Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection
- PMID: 17409164
- PMCID: PMC1900112
- DOI: 10.1128/JVI.00239-07
Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection
Abstract
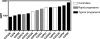
The study of the evolution and specificities of neutralizing antibodies during the course of human immunodeficiency virus type 1 (HIV-1) infection may be important in the discovery of possible targets for vaccine design. In this study, we assessed the autologous and heterologous neutralization responses of 14 HIV-1 subtype C-infected individuals, using envelope clones obtained within the first 2 months postinfection. Our data show that potent but relatively strain-specific neutralizing antibodies develop within 3 to 12 months of HIV-1 infection. The magnitude of this response was associated with shorter V1-to-V5 envelope lengths and fewer glycosylation sites, particularly in the V1-V2 region. Anti-MPER antibodies were detected in 4 of 14 individuals within a year of infection, while antibodies to CD4-induced (CD4i) epitopes developed to high titers in 12 participants, in most cases before the development of autologous neutralizing antibodies. However, neither anti-MPER nor anti-CD4i antibody specificity conferred neutralization breadth. These data provide insights into the kinetics, potency, breadth, and epitope specificity of neutralizing antibody responses in acute HIV-1 subtype C infection.
Figures




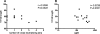



References
-
- Decker, J. M., F. Bibollet-Ruche, X. Wei, S. Wang, D. N. Levy, W. Wang, E. Delaporte, M. Peeters, C. A. Derdeyn, S. Allen, E. Hunter, M. S. Saag, J. A. Hoxie, B. H. Hahn, P. D. Kwong, J. E. Robinson, and G. M. Shaw. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407-1419. - PMC - PubMed
-
- Gao, F., S. G. Morrison, D. L. Robertson, C. L. Thornton, S. Craig, G. Karlsson, J. Sodroski, M. Morgado, B. Galvao-Castro, H. von Briesen, S. Beddows, J. Weber, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 1996. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. J. Virol. 70:1651-1667. - PMC - PubMed
-
- Haynes, B. F., J. Fleming, E. W. St. Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

