Removal of GABA within adult modulatory systems alters electrical coupling and allows expression of an embryonic-like network
- PMID: 17409227
- PMCID: PMC6672421
- DOI: 10.1523/JNEUROSCI.4406-06.2007
Removal of GABA within adult modulatory systems alters electrical coupling and allows expression of an embryonic-like network
Abstract
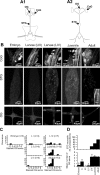
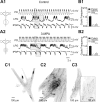
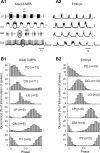
The maturation and operation of neural networks are known to depend on modulatory neurons. However, whether similar mechanisms may control both adult and developmental plasticity remains poorly investigated. To examine this issue, we have used the lobster stomatogastric nervous system (STNS) to investigate the ontogeny and role of GABAergic modulatory neurons projecting to small pattern generating networks. Using immunocytochemistry, we found that modulatory input neurons to the stomatogastric ganglion (STG) express GABA only after metamorphosis, a time that coincides with the developmental switch from a single to multiple pattern generating networks within the STNS. We demonstrate that blocking GABA synthesis with 3-mercapto-propionic acid within the adult modulatory neurons results in the reconfiguration of the distinct STG networks into a single network that generates a unified embryonic-like motor pattern. Using dye-coupling experiments, we also found that gap-junctional coupling is greater in embryos and GABA-deprived adults exhibiting the unified motor pattern compared with control adults. Furthermore, GABA was found to diminish directly the extent and strength of electrical coupling within adult STG networks. Together, these observations suggest the acquisition of a GABAergic phenotype by modulatory neurons after metamorphosis may induce the reconfiguration of the single embryonic network into multiple adult networks by directly decreasing electrical coupling. The findings also suggest that adult neural networks retain the ability to express typical embryonic characteristics, indicating that network ontogeny can be reversed and that changes in electrical coupling during development may allow the segregation of multiple distinct functional networks from a single large embryonic network.
Figures







Similar articles
-
Ontogeny of modulatory inputs to motor networks: early established projection and progressive neurotransmitter acquisition.J Neurosci. 2001 Feb 15;21(4):1313-26. doi: 10.1523/JNEUROSCI.21-04-01313.2001. J Neurosci. 2001. PMID: 11160402 Free PMC article.
-
Central inputs mask multiple adult neural networks within a single embryonic network.Nature. 1999 Dec 9;402(6762):660-4. doi: 10.1038/45238. Nature. 1999. PMID: 10604471
-
Maturation of rhythmic neural network: role of central modulatory inputs.J Physiol Paris. 2003 Jan;97(1):59-68. doi: 10.1016/j.jphysparis.2003.10.007. J Physiol Paris. 2003. PMID: 14706691 Review.
-
Innexins in the lobster stomatogastric nervous system: cloning, phylogenetic analysis, developmental changes and expression within adult identified dye and electrically coupled neurons.Eur J Neurosci. 2006 Dec;24(11):3119-33. doi: 10.1111/j.1460-9568.2006.05209.x. Eur J Neurosci. 2006. PMID: 17156373
-
Development of the peptidergic modulation of a rhythmic pattern generating network.Brain Res. 1999 Nov 27;848(1-2):35-44. doi: 10.1016/s0006-8993(99)02118-6. Brain Res. 1999. PMID: 10612696 Review.
Cited by
-
Molecular characterization of putative neuropeptide, amine, diffusible gas and small molecule transmitter biosynthetic enzymes in the eyestalk ganglia of the American lobster, Homarus americanus.Invert Neurosci. 2018 Oct 1;18(4):12. doi: 10.1007/s10158-018-0216-4. Invert Neurosci. 2018. PMID: 30276482 Free PMC article.
-
Complicating connectomes: Electrical coupling creates parallel pathways and degenerate circuit mechanisms.Dev Neurobiol. 2017 May;77(5):597-609. doi: 10.1002/dneu.22410. Epub 2016 Aug 8. Dev Neurobiol. 2017. PMID: 27314561 Free PMC article. Review.
-
Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus.J Neurosci. 2008 Sep 24;28(39):9828-39. doi: 10.1523/JNEUROSCI.2328-08.2008. J Neurosci. 2008. PMID: 18815267 Free PMC article.
-
Electrical coupling and innexin expression in the stomatogastric ganglion of the crab Cancer borealis.J Neurophysiol. 2014 Dec 1;112(11):2946-58. doi: 10.1152/jn.00536.2014. Epub 2014 Sep 10. J Neurophysiol. 2014. PMID: 25210156 Free PMC article.
-
Postembryonic development of centrally generated flight motor patterns in the hawkmoth, Manduca sexta.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Jan;196(1):37-50. doi: 10.1007/s00359-009-0490-z. Epub 2009 Nov 19. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 19924416
References
-
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–1726. - PubMed
-
- Bem T, Le Feuvre Y, Simmers J, Meyrand P. Electrical coupling can prevent expression of adult-like properties in an embryonic neural circuit. J Neurophysiol. 2002;87:538–547. - PubMed
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
