T-type and L-type Ca2+ conductances define and encode the bimodal firing pattern of vestibulocerebellar unipolar brush cells
- PMID: 17409247
- PMCID: PMC6672410
- DOI: 10.1523/JNEUROSCI.4719-06.2007
T-type and L-type Ca2+ conductances define and encode the bimodal firing pattern of vestibulocerebellar unipolar brush cells
Abstract
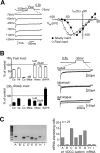
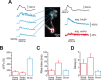
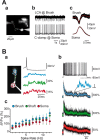
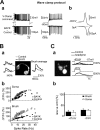
Cerebellar unipolar brush cells (UBCs) are glutamatergic interneurons that receive direct input from vestibular afferents in the form of a unique excitatory synapse on their dendritic brush. UBCs constitute independent relay lines for vestibular signals, and their inherent properties most likely determine how vestibular activity is encoded by the cerebellar cortex. We now demonstrate that UBCs are bimodal cells; they can either fire high-frequency bursts of action potentials when stimulated from hyperpolarized potentials or discharge tonically during sustained depolarizations. The two functional states can be triggered by physiological-like activity of the excitatory input and are encoded by distinct Ca2+-signaling systems. By combining complementary strategies, consisting of molecular and electrophysiological analysis and of ultrafast acousto-optical deflector-based two-photon imaging, we unraveled the identity and the subcellular localization of the Ca2+ conductances activating in each mode. Fast inactivating T-type Ca2+ channels produce low-threshold spikes, which trigger the high-frequency bursts and generate powerful Ca2+ transients in the brush and, to a much lesser extent, in the soma. The tonic firing mode is encoded by a signalization system principally composed of L-type channels. Ca2+ influx during tonic firing produces a linear representation of the spike rate of the cell in the form of a widespread and sustained Ca2+ concentration increase and regulates cellular excitability via BK potassium channels. The bimodal firing pattern of UBCs may underlie different coding strategies of the vestibular input by the cerebellum, thus likely increasing the computational power of this structure.
Figures



References
-
- Altman J, Bayer SA. Time of origin and distribution of a new cell type in the rat cerebellar cortex. Exp Brain Res. 1977;29:265–274. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Barbour B, Isope P. Combining loose cell-attached stimulation and recording. J Neurosci Methods. 2000;103:199–208. - PubMed
-
- Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
