Brain oscillations control timing of single-neuron activity in humans
- PMID: 17409248
- PMCID: PMC6672400
- DOI: 10.1523/JNEUROSCI.4636-06.2007
Brain oscillations control timing of single-neuron activity in humans
Abstract
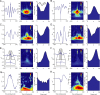
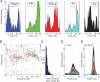
A growing body of animal research suggests that neurons represent information not only in terms of their firing rates but also by varying the timing of spikes relative to neuronal oscillations. Although researchers have argued that this temporal coding is critical in human memory and perception, no supporting data from humans have been reported. This study provides the first analysis of the temporal relationship between brain oscillations and single-neuron activity in humans. Recording from 1924 neurons, we find that neuronal activity in various brain regions increases at specific phases of brain oscillations. Neurons in widespread brain regions were phase locked to oscillations in the theta- (4-8 Hz) and gamma- (30-90 Hz) frequency bands. In hippocampus, phase locking was prevalent in the delta- (1-4 Hz) and gamma-frequency bands. Individual neurons were phase locked to various phases of theta and delta oscillations, but they only were active at the trough of gamma oscillations. These findings provide support for the temporal-coding hypothesis in humans. Specifically, they indicate that theta and delta oscillations facilitate phase coding and that gamma oscillations help to decode combinations of simultaneously active neurons.
Figures

References
-
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. - PubMed
-
- Bódizs R, Kántor S, Szabó G, Szũcs A, Erõss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001:747–753. - PubMed
-
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. - PubMed
-
- Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
