Plexin-B2 controls the development of cerebellar granule cells
- PMID: 17409257
- PMCID: PMC6672405
- DOI: 10.1523/JNEUROSCI.4710-06.2007
Plexin-B2 controls the development of cerebellar granule cells
Abstract
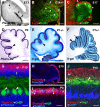
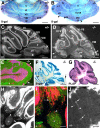
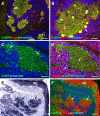
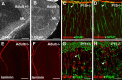
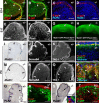
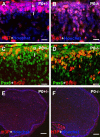
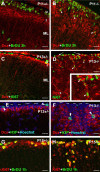
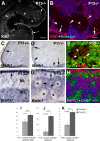
Cerebellar granule cell progenitors proliferate postnatally in the upper part of the external granule cell layer (EGL) of the cerebellum. Postmitotic granule cells differentiate and migrate, tangentially in the EGL and then radially through the molecular and Purkinje cell layers. The molecular control of the transition between proliferation and differentiation in cerebellar granule cells is poorly understood. We show here that the transmembrane receptor Plexin-B2 is expressed by proliferating granule cell progenitors. To study Plexin-B2 function, we generated a targeted mutation of mouse Plexin-B2. Most Plexin-B2(-/-) mutants die at birth as a result of neural tube closure defects. Some mutants survive but their cerebellum cytoarchitecture is profoundly altered. This is correlated with a disorganization of the timing of granule cell proliferation and differentiation in the EGL. Many differentiated granule cells migrate inside the cerebellum and keep proliferating. These results reveal that Plexin-B2 controls the balance between proliferation and differentiation in granule cells.
Figures
Similar articles
-
Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo.J Neurosci. 2007 Jun 6;27(23):6333-47. doi: 10.1523/JNEUROSCI.5381-06.2007. J Neurosci. 2007. PMID: 17554007 Free PMC article.
-
Semaphorin 4C and 4G are ligands of Plexin-B2 required in cerebellar development.Mol Cell Neurosci. 2011 Feb;46(2):419-31. doi: 10.1016/j.mcn.2010.11.005. Epub 2010 Nov 29. Mol Cell Neurosci. 2011. PMID: 21122816 Free PMC article.
-
Plexin-B2 controls the timing of differentiation and the motility of cerebellar granule neurons.Elife. 2021 Jun 8;10:e60554. doi: 10.7554/eLife.60554. Elife. 2021. PMID: 34100719 Free PMC article.
-
DNER as key molecule for cerebellar maturation.Cerebellum. 2006;5(3):227-31. doi: 10.1080/14734220600632564. Cerebellum. 2006. PMID: 16997755 Review.
-
The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma.Mol Brain. 2015 Oct 17;8(1):64. doi: 10.1186/s13041-015-0155-5. Mol Brain. 2015. PMID: 26475605 Free PMC article. Review.
Cited by
-
Plexin-mediated neuronal development and neuroinflammatory responses in the nervous system.Histol Histopathol. 2023 Nov;38(11):1239-1248. doi: 10.14670/HH-18-625. Epub 2023 Apr 25. Histol Histopathol. 2023. PMID: 37170703 Review.
-
Plexin-B2 Mediates Physiologic and Pathologic Functions of Angiogenin.Cell. 2017 Nov 2;171(4):849-864.e25. doi: 10.1016/j.cell.2017.10.005. Cell. 2017. PMID: 29100074 Free PMC article.
-
Plexin-B2 facilitates glioblastoma infiltration by modulating cell biomechanics.Commun Biol. 2021 Jan 29;4(1):145. doi: 10.1038/s42003-021-01667-4. Commun Biol. 2021. PMID: 33514835 Free PMC article.
-
Invasion of glioma cells through confined space requires membrane tension regulation and mechano-electrical coupling via Plexin-B2.Nat Commun. 2025 Jan 2;16(1):272. doi: 10.1038/s41467-024-55056-6. Nat Commun. 2025. PMID: 39747004 Free PMC article.
-
Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions.Cell Mol Life Sci. 2012 Nov;69(22):3765-805. doi: 10.1007/s00018-012-1019-0. Epub 2012 Jun 29. Cell Mol Life Sci. 2012. PMID: 22744749 Free PMC article. Review.
References
-
- Alder J, Lee KJ, Jessell TM, Hatten ME. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2:535–540. - PubMed
-
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
