Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing
- PMID: 17409308
- PMCID: PMC1978101
- DOI: 10.1681/ASN.2006111263
Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing
Abstract
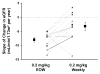
This study was performed to determine whether adult male patients with Fabry disease who demonstrate a continuing decline in renal function despite 2 to 4 yr of conventionally dosed agalsidase alfa therapy (0.2 mg/kg every other week [EOW]) show an improved slope of decline with weekly administration using the same dosage. Eleven (27%) of 41 adult male patients with Fabry disease who participated in long-term agalsidase alfa clinical trials and who had demonstrated a slope of decline in estimated GFR (eGFR) of > or =5 ml/min per 1.73 m(2)/yr while receiving long-term treatment with agalsidase alfa at the currently recommended dosage of 0.2 mg/kg, infused EOW, were enrolled in this open-label, prospective study. Patients were switched from EOW to weekly infusions and followed for an additional 24 mo. Before switching to weekly dosing, eGFR was 53.7 +/- 6.3 ml/min per 1.73 m(2) (mean +/- SEM), and mean rate of change in eGFR was -8.0 +/- 0.8 ml/min per 1.73 m(2)/yr. During the 24-mo follow-up period after switching to weekly dosing, the mean rate of change in eGFR was observed to slow to -3.3 +/- 1.4 ml/min/1.73 m(2)/yr (P = 0.01 versus EOW). After switching to weekly dosing, three patients demonstrated an improvement in eGFR and six patients demonstrated a slowing in the rate of eGFR decline; only two patients failed to improve their eGFR slope. A multiple regression model confirmed that the weekly infusion regimen was the strongest explanatory variable for the change in eGFR (P = 0.0008), with a weaker contribution from the concomitant use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers (P = 0.02). These results suggest that weekly infusions of agalsidase alfa at a dosage of 0.2 mg/kg may be beneficial in the subgroup of patients who have Fabry disease and whose kidney function continues to decline after 2 to 4 yr or more of standard EOW dosing.
Figures


Comment in
-
Enzyme replacement therapy and Fabry kidney disease: quo vadis?J Am Soc Nephrol. 2007 May;18(5):1368-70. doi: 10.1681/ASN.2007030312. Epub 2007 Apr 11. J Am Soc Nephrol. 2007. PMID: 17429046 No abstract available.
References
-
- Brady R, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. - PubMed
-
- Brady RO, Schiffmann R. Clinical features of and recent advances in therapy for Fabry disease. JAMA. 2000;284:2771–2775. - PubMed
-
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. - PubMed
-
- Ries M, Moore DF, Robinson CJ, Tifft CJ, Rosenbaum KN, Brady RO, Schiffmann R, Krasnewich D. Quantitative dys-morphology assessment in Fabry disease. Genet Med. 2006;8:96–101. - PubMed
-
- Ries M, Ramaswami U, Parini R, Lindblad B, Whybra C, Willers I, Gal A, Beck M. The early clinical phenotype of Fabry disease: A study on 35 European children and adolescents. Eur J Pediatr. 2003;162:767–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

