Trophic factor induction of human umbilical cord blood cells in vitro and in vivo
- PMID: 17409487
- PMCID: PMC2680126
- DOI: 10.1088/1741-2560/4/2/013
Trophic factor induction of human umbilical cord blood cells in vitro and in vivo
Abstract
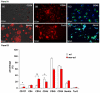
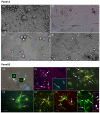
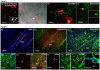
The mononuclear fraction of human umbilical cord blood (HUCBmnf) is a mixed cell population that multiple research groups have shown contains cells that can express neural proteins. In these studies, we have examined the ability of the HUCBmnf to express neural antigens after in vitro exposure to defined media supplemented with a cocktail of growth and neurotrophic factors. It is our hypothesis that by treating the HUCBmnf with these developmentally-relevant factors, we can expand the population, enhance the expression of neural antigens and increase cell survival upon transplantation. Prior to growth factor treatment in culture, expression of stem cell antigens is greater in the non-adherent HUCBmnf cells compared to the adherent cells (p < 0.05). Furthermore, treatment of the non-adherent cells with growth factors, increases BrdU incorporation, especially after 14 days in vitro (DIV). In HUCBmnf-embryonic mouse striata co-culture, a small number of growth factor treated HUCBmnf cells were able to integrate into the growing neural network and express immature (nestin and TuJ1) and mature (GFAP and MAP2) neural markers. Treated HUCBmnf cells implanted in the subventricular zone predominantly expressed GFAP although some grafted HUCBmnf cells were MAP2 positive. While short-term treatment of HUCBmnf cells with growth and neurotrophic factors enhanced proliferative capacity in vitro and survival of the cells in vivo, the treatment regimen employed was not enough to ensure long-term survival of HUCBmnf-derived neurons necessary for cell replacement therapies for neurodegenerative diseases.
Figures


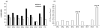
References
-
- Kim SY, Park SY, Kim JM, Kim JW, Kim MY, Yang JH, Kim JO, Choi KH, Kim SB, Ryu HM. Differentiation of endothelial cells from human umbilical cord blood AC133-CD14+ cells. Ann. Hematol. 2005;84:417–22. - PubMed
-
- Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol. Blood Marrow Transplant. 2001;7:581–8. - PubMed
-
- Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005;7:209–18. - PubMed
-
- Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif. Tissue Int. 2003;72:135–42. - PubMed
-
- Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004;22:981–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
