GM-CSF-secreting cancer immunotherapies: preclinical analysis of the mechanism of action
- PMID: 17410360
- PMCID: PMC11029840
- DOI: 10.1007/s00262-007-0315-2
GM-CSF-secreting cancer immunotherapies: preclinical analysis of the mechanism of action
Abstract
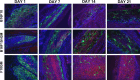
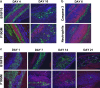
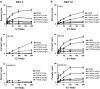
Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting tumor cell immunotherapies have demonstrated long-lasting, and specific anti-tumor immune responses in animal models. The studies reported here specifically evaluate two aspects of the immune response generated by such immunotherapies: the persistence of irradiated tumor cells at the immunization site, and the breadth of the immune response elicited to tumor associated antigens (TAA) derived from the immunotherapy. To further define the mechanism of GM-CSF-secreting cancer immunotherapies, immunohistochemistry studies were performed using the B16F10 melanoma tumor model. In contrast to previous reports, our data revealed that the irradiated tumor cells persisted and secreted high levels of GM-CSF at the injection site for more than 21 days. Furthermore, dense infiltrates of dendritic cells were observed only in mice treated with GM-CSF-secreting B16F10 cells, and not in mice treated with unmodified B16F10 cells with or without concurrent injection of rGM-CSF. In addition, histological studies also revealed enhanced neutrophil and CD4+ T cell infiltration, as well as the presence of apoptotic cells, at the injection site of mice treated with GM-CSF-secreting tumor cells. To evaluate the scope of the immune response generated by GM-CSF-secreting cancer immunotherapies, several related B16 melanoma tumor cell subclones that exist as a result of genetic drift in the original cell line were used to challenge mice previously immunized with GM-CSF-secreting B16F10 cells. These studies revealed that GM-CSF-secreting cancer immunotherapies elicit T cell responses that effectively control growth of related but antigenically distinct tumors. Taken together, these studies provide important new insights into the mechanism of action of this promising novel cancer immunotherapy.
Figures



Similar articles
-
Allogeneic GM-CSF-secreting tumor cell immunotherapies generate potent anti-tumor responses comparable to autologous tumor cell immunotherapies.Clin Immunol. 2009 Nov;133(2):184-97. doi: 10.1016/j.clim.2009.07.008. Epub 2009 Aug 7. Clin Immunol. 2009. PMID: 19664962
-
The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration.Cancer Immunol Immunother. 2006 Oct;55(10):1285-93. doi: 10.1007/s00262-005-0116-4. Epub 2006 Jan 12. Cancer Immunol Immunother. 2006. PMID: 16408214 Free PMC article.
-
Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb.Clin Immunol. 2007 Oct;125(1):76-87. doi: 10.1016/j.clim.2007.07.005. Epub 2007 Aug 13. Clin Immunol. 2007. PMID: 17706463
-
GM-CSF-secreting vaccines for solid tumors: moving forward.Discov Med. 2010 Jul;10(50):52-60. Discov Med. 2010. PMID: 20670599 Free PMC article. Review.
-
Concise review: engineering the fusion of cytokines for the modulation of immune cellular responses in cancer and autoimmune disorders.Stem Cells Transl Med. 2015 Jan;4(1):66-73. doi: 10.5966/sctm.2014-0145. Epub 2014 Nov 12. Stem Cells Transl Med. 2015. PMID: 25391644 Free PMC article. Review.
Cited by
-
Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity.Clin Exp Metastasis. 2009;26(3):179-87. doi: 10.1007/s10585-008-9228-5. Epub 2008 Dec 14. Clin Exp Metastasis. 2009. PMID: 19082918
-
Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model.PLoS One. 2017 May 25;12(5):e0178479. doi: 10.1371/journal.pone.0178479. eCollection 2017. PLoS One. 2017. PMID: 28542574 Free PMC article.
-
Oncolytic viruses for cancer immunotherapy.J Hematol Oncol. 2020 Jun 29;13(1):84. doi: 10.1186/s13045-020-00922-1. J Hematol Oncol. 2020. PMID: 32600470 Free PMC article. Review.
-
Combination immunotherapy and active-specific tumor cell vaccination augments anti-cancer immunity in a mouse model of gastric cancer.J Transl Med. 2011 Aug 22;9:140. doi: 10.1186/1479-5876-9-140. J Transl Med. 2011. PMID: 21859450 Free PMC article.
-
Oncolytic adenovirus inhibits TNBC tumor growth/metastasis in mice by targeting TGF-β and overexpressing GM-CSF.Mol Ther Oncol. 2025 Jan 17;33(1):200936. doi: 10.1016/j.omton.2025.200936. eCollection 2025 Mar 20. Mol Ther Oncol. 2025. PMID: 40104170 Free PMC article.
References
-
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. - DOI - PMC - PubMed
-
- Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte–macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. - PubMed
-
- Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

