ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia
- PMID: 17411338
- PMCID: PMC1847689
- DOI: 10.1371/journal.ppat.0030046
ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia
Abstract
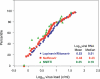
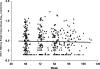
Current antiretroviral therapy is effective in suppressing but not eliminating HIV-1 infection. Understanding the source of viral persistence is essential for developing strategies to eradicate HIV-1 infection. We therefore investigated the level of plasma HIV-1 RNA in patients with viremia suppressed to less than 50-75 copies/ml on standard protease inhibitor- or non-nucleoside reverse transcriptase inhibitor-containing antiretroviral therapy using a new, real-time PCR-based assay for HIV-1 RNA with a limit of detection of one copy of HIV-1 RNA. Single copy assay results revealed that >80% of patients on initial antiretroviral therapy for 60 wk had persistent viremia of one copy/ml or more with an overall median of 3.1 copies/ml. The level of viremia correlated with pretherapy plasma HIV-1 RNA but not with the specific treatment regimen. Longitudinal studies revealed no significant decline in the level of viremia between 60 and 110 wk of suppressive antiretroviral therapy. These data suggest that the persistent viremia on current antiretroviral therapy is derived, at least in part, from long-lived cells that are infected prior to initiation of therapy.
Conflict of interest statement
Figures


References
-
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. - PubMed
-
- Pomerantz RJ, Zhang H. Residual HIV-1 persistence during suppressive HAART. Curr Clin Top Infect Dis. 2001;21:1–30. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

