M. tuberculosis Ser/Thr protein kinase D phosphorylates an anti-anti-sigma factor homolog
- PMID: 17411339
- PMCID: PMC1847690
- DOI: 10.1371/journal.ppat.0030049
M. tuberculosis Ser/Thr protein kinase D phosphorylates an anti-anti-sigma factor homolog
Abstract
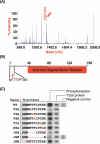
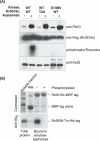
Receptor Ser/Thr protein kinases are candidates for sensors that govern developmental changes and disease processes of Mycobacterium tuberculosis (Mtb), but the functions of these kinases are not established. Here, we show that Mtb protein kinase (Pkn) D overexpression alters transcription of numerous bacterial genes, including Rv0516c, a putative anti-anti-sigma factor, and genes regulated by sigma factor F. The PknD kinase domain directly phosphorylated Rv0516c, but no other sigma factor regulator, in vitro. In contrast, the purified PknB and PknE kinase domains phosphorylated distinct sigma regulators. Rather than modifying a consensus site, PknD phosphorylated Rv0516c in vitro and in vivo on Thr2 in a unique N-terminal extension. This phosphorylation inhibited Rv0516c binding in vitro to a homologous anti-anti-sigma factor, Rv2638. These results support a model in which signals transmitted through PknD alter the transcriptional program of Mtb by stimulating phosphorylation of a sigma factor regulator at an unprecedented control site.
Conflict of interest statement
Figures



References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. - PubMed
-
- Zhang Y. Persistent and dormant tubercle bacilli and latent tuberculosis. Front Biosci. 2004;9:1136–1156. - PubMed
-
- Russell DG. Mycobacterium tuberculosis: Here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. - PubMed
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
-
- Av-Gay Y, Everett M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis . Trends Microbiol. 2000;8:238–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

