Integration of light and temperature in the regulation of circadian gene expression in Drosophila
- PMID: 17411344
- PMCID: PMC1847695
- DOI: 10.1371/journal.pgen.0030054
Integration of light and temperature in the regulation of circadian gene expression in Drosophila
Abstract
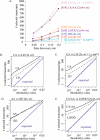
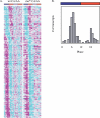
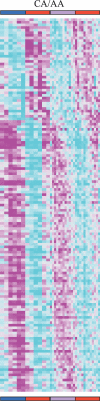
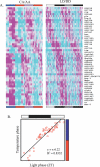
Circadian clocks are aligned to the environment via synchronizing signals, or Zeitgebers, such as daily light and temperature cycles, food availability, and social behavior. In this study, we found that genome-wide expression profiles from temperature-entrained flies show a dramatic difference in the presence or absence of a thermocycle. Whereas transcript levels appear to be modified broadly by changes in temperature, there is a specific set of temperature-entrained circadian mRNA profiles that continue to oscillate in constant conditions. There are marked differences in the biological functions represented by temperature-driven or circadian regulation. The set of temperature-entrained circadian transcripts overlaps significantly with a previously defined set of transcripts oscillating in response to a photocycle. In follow-up studies, all thermocycle-entrained circadian transcript rhythms also responded to light/dark entrainment, whereas some photocycle-entrained rhythms did not respond to temperature entrainment. Transcripts encoding the clock components Period, Timeless, Clock, Vrille, PAR-domain protein 1, and Cryptochrome were all confirmed to be rhythmic after entrainment to a daily thermocycle, although the presence of a thermocycle resulted in an unexpected phase difference between period and timeless expression rhythms at the transcript but not the protein level. Generally, transcripts that exhibit circadian rhythms both in response to thermocycles and photocycles maintained the same mutual phase relationships after entrainment by temperature or light. Comparison of the collective temperature- and light-entrained circadian phases of these transcripts indicates that natural environmental light and temperature cycles cooperatively entrain the circadian clock. This interpretation is further supported by comparative analysis of the circadian phases observed for temperature-entrained and light-entrained circadian locomotor behavior. Taken together, these findings suggest that information from both light and temperature is integrated by the transcriptional clock mechanism in the adult fly head.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures



References
-
- Hardin PE. The circadian timekeeping system of Drosophila . Curr Biol. 2005;15:R714–R722. - PubMed
-
- Chang DC, Reppert SM. A novel C-terminal domain of Drosophila Period inhibits dClock:Cycle-mediated transcription. Curr Biol. 2003;13:758–762. - PubMed
-
- Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, et al. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. - PubMed
-
- Glossop NRJ, Houl JH, Zheng H, Ng FS, Dudek SM, et al. Vrille feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

