1alpha,25-Dihydroxyvitamin D(3) antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21(waf1) upregulation in human osteosarcoma
- PMID: 17412493
- PMCID: PMC2760385
- DOI: 10.1016/j.canlet.2007.02.013
1alpha,25-Dihydroxyvitamin D(3) antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21(waf1) upregulation in human osteosarcoma
Abstract
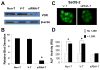
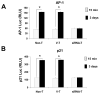
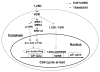
The molecular mechanisms underlying antiproliferative actions of the steroid 1alpha,25-dihydroxy vitamin D(3) (1,25D) in human osteosarcoma cells are known only partially. To better understand the signaling involved in 1,25D anti-tumorigenic properties in bone, we stably silenced vitamin D receptor (VDR) expression in the human osteosarcoma SaOS-2 cell line. We found that 1,25D treatment reduced cell proliferation by approximately 25% after 3 days only in SaOS-2 cells expressing native levels of VDR protein, and involved activation of MAPK/AP-1/p21(waf1) pathways. Both sustained (3 days) and transient (15min) 1,25D treatment activated JNK and ERK1/2 MAPK signaling in a nongenomic VDR-dependent manner. However, only sustained exposure to hormone led to upregulation of p21 and subsequent genomic control of the cell cycle. Specific blockade of MEK1/MEK2 cascade upstream from ERK1/2 abrogated 1,25D activation of AP-1 and p21, and subsequent antiproliferative effects, even in the presence of a nuclear VDR. We conclude that 1,25D-induced inhibition of human osteosarcoma cell proliferation occurs via sustained activation of JNK and MEK1/MEK2 pathways downstream of nongenomic VDR signaling that leads to upregulation of a c-Jun/c-Fos (AP-1) complex, which in turn modulates p21(waf1) gene expression. Our results demonstrate a cross-talk between 1,25D/VDR nongenomic and genomic signaling at the level of MAP kinase activation that leads to reduction of cell proliferation in human osteosarcoma cells.
Figures



Similar articles
-
[The role of mitogen-activated protein kinase cascades in inhibition of proliferation in human prostate carcinoma cells by raloxifene: an in vitro experiment].Zhonghua Yi Xue Za Zhi. 2008 Jan 22;88(4):271-5. Zhonghua Yi Xue Za Zhi. 2008. PMID: 18361842 Chinese.
-
The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition.J Biol Chem. 2002 Jul 19;277(29):25884-92. doi: 10.1074/jbc.M203039200. Epub 2002 Apr 30. J Biol Chem. 2002. PMID: 11983707
-
1α,25 dihydroxi-vitamin D₃ modulates CDK4 and CDK6 expression and localization.Biochem Biophys Res Commun. 2015 Mar 27;459(1):137-42. doi: 10.1016/j.bbrc.2015.02.083. Epub 2015 Feb 23. Biochem Biophys Res Commun. 2015. PMID: 25721671
-
Role of VDR in 1α,25-dihydroxyvitamin D3-dependent non-genomic activation of MAPKs, Src and Akt in skeletal muscle cells.J Steroid Biochem Mol Biol. 2013 Jul;136:125-30. doi: 10.1016/j.jsbmb.2013.02.013. Epub 2013 Mar 5. J Steroid Biochem Mol Biol. 2013. PMID: 23470620 Review.
-
Cellular and molecular effects of vitamin D on carcinogenesis.Arch Biochem Biophys. 2012 Jul 1;523(1):107-14. doi: 10.1016/j.abb.2011.10.019. Epub 2011 Nov 9. Arch Biochem Biophys. 2012. PMID: 22085499 Free PMC article. Review.
Cited by
-
Enhanced Antiproliferative Effect of Combined Treatment with Calcitriol and All-Trans Retinoic Acid in Relation to Vitamin D Receptor and Retinoic Acid Receptor α Expression in Osteosarcoma Cell Lines.Int J Mol Sci. 2020 Sep 9;21(18):6591. doi: 10.3390/ijms21186591. Int J Mol Sci. 2020. PMID: 32916897 Free PMC article.
-
What are nuclear receptor ligands?Mol Cell Endocrinol. 2011 Mar 1;334(1-2):3-13. doi: 10.1016/j.mce.2010.06.018. Epub 2010 Jul 6. Mol Cell Endocrinol. 2011. PMID: 20615454 Free PMC article. Review.
-
Vitamin D and cancer: a review of molecular mechanisms.Biochem J. 2012 Jan 1;441(1):61-76. doi: 10.1042/BJ20110744. Biochem J. 2012. PMID: 22168439 Free PMC article. Review.
-
Stimulators of mineralization limit the invasive phenotype of human osteosarcoma cells by a mechanism involving impaired invadopodia formation.PLoS One. 2014 Oct 14;9(10):e109938. doi: 10.1371/journal.pone.0109938. eCollection 2014. PLoS One. 2014. PMID: 25314307 Free PMC article.
-
Activation of rapid signaling pathways does not contribute to 1 alpha,25-dihydroxyvitamin D3-induced growth inhibition of mouse prostate epithelial progenitor cells.J Cell Biochem. 2009 Aug 1;107(5):1031-6. doi: 10.1002/jcb.22206. J Cell Biochem. 2009. PMID: 19492419 Free PMC article.
References
-
- Butterwo CE. Vitamin deficiency and cancer. Med Oncol. 1985:T165–174. - PubMed
-
- Binderup L. Vitamin D analogues: New regulators of cancer cell growth and differentiation. Bioorg Med Chem Lett. 1993;3:1891–1896.
-
- Matsumoto T, Sowa Y, Ohtani-Fujita N, et al. p53-independent induction of WAF1/Cip1 is correlated with osteoblastic differentiation by vitamin D3. Cancer Lett. 1998;129:61–68. - PubMed
-
- Witasp E, Gustafsson AC, Cotgreave I, Lind M, Fadeel B. Vitamin D fails to prevent serum starvation- or staurosporine-induced apoptosis in human and rat osteosarcoma-derived cell lines. Biochem Biophys Res Comm. 2005;330:891–897. - PubMed
-
- Barroga EF, Kadosawa T, Okumura M, Fujinaga T. Effects of vitamin D and retinoids on the differentiation and growth in vitro of canine osteosarcoma and its clonal cell lines. Res Vet Sci. 1999;66:231–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

