Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae
- PMID: 17412552
- PMCID: PMC1950449
- DOI: 10.1016/j.micpath.2007.02.004
Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae
Abstract
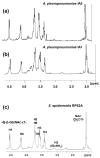
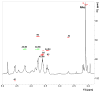
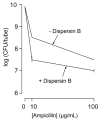
Most field isolates of the swine pathogen Actinobacillus pleuropneumoniae form tenacious biofilms on abiotic surfaces in vitro. We purified matrix polysaccharides from biofilms produced by A. pleuropneumoniae field isolates IA1 and IA5 (serotypes 1 and 5, respectively), and determined their chemical structures by using NMR spectroscopy. Both strains produced matrix polysaccharides consisting of linear chains of N-acetyl-D-glucosamine (GlcNAc) residues in beta(1,6) linkage (poly-beta-1,6-GlcNAc or PGA). A small percentage of the GlcNAc residues in each polysaccharide were N-deacetylated. These structures were nearly identical to those of biofilm matrix polysaccharides produced by Escherichia coli, Staphylococcus aureus and Staphylococcus epidermidis. PCR analyses indicated that a gene encoding the PGA-specific glycoside transferase enzyme PgaC was present on the chromosome of 15 out of 15 A. pleuropneumoniae reference strains (serotypes 1-12) and 76 out of 77 A. pleuropneumoniae field isolates (serotypes 1, 5 and 7). A pgaC mutant of strain IA5 failed to form biofilms in vitro, as did wild-type strains IA1 and IA5 when grown in broth supplemented with the PGA-hydrolyzing enzyme dispersin B. Treatment of IA5 biofilms with dispersin B rendered them more sensitive to killing by ampicillin. Our findings suggest that PGA functions as a major biofilm adhesin in A. pleuropneumoniae. Biofilm formation may have relevance to the colonization and pathogenesis of A. pleuropneumoniae in pigs.
Figures


Similar articles
-
Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms.J Bacteriol. 2004 Dec;186(24):8213-20. doi: 10.1128/JB.186.24.8213-8220.2004. J Bacteriol. 2004. PMID: 15576769 Free PMC article.
-
Sub-inhibitory concentrations of penicillin G induce biofilm formation by field isolates of Actinobacillus pleuropneumoniae.Vet Microbiol. 2015 Sep 30;179(3-4):277-86. doi: 10.1016/j.vetmic.2015.06.011. Epub 2015 Jun 24. Vet Microbiol. 2015. PMID: 26130517
-
Effects of growth conditions on biofilm formation by Actinobacillus pleuropneumoniae.Vet Res. 2010 Jan-Feb;41(1):3. doi: 10.1051/vetres/2009051. Epub 2009 Sep 10. Vet Res. 2010. PMID: 19737507 Free PMC article.
-
Actinobacillus pleuropneumoniae biofilms: Role in pathogenicity and potential impact for vaccination development.Anim Health Res Rev. 2018 Jun;19(1):17-30. doi: 10.1017/S146625231700010X. Epub 2017 Nov 7. Anim Health Res Rev. 2018. PMID: 29110751 Review.
-
Surface polysaccharides and iron-uptake systems of Actinobacillus pleuropneumoniae.Can J Vet Res. 2004 Apr;68(2):81-5. Can J Vet Res. 2004. PMID: 15188950 Free PMC article. Review.
Cited by
-
Noneluting enzymatic antibiofilm coatings.ACS Appl Mater Interfaces. 2012 Sep 26;4(9):4708-16. doi: 10.1021/am3010847. Epub 2012 Sep 4. ACS Appl Mater Interfaces. 2012. PMID: 22909396 Free PMC article.
-
(1)H NMR-based metabolite profiling of planktonic and biofilm cells in Acinetobacter baumannii 1656-2.PLoS One. 2013;8(3):e57730. doi: 10.1371/journal.pone.0057730. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483923 Free PMC article.
-
Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host.Vet Res. 2010 Sep-Oct;41(5):65. doi: 10.1051/vetres/2010037. Epub 2010 Jun 15. Vet Res. 2010. PMID: 20546697 Free PMC article. Review.
-
Enhancing the utility of existing antibiotics by targeting bacterial behaviour?Br J Pharmacol. 2012 Feb;165(4):845-57. doi: 10.1111/j.1476-5381.2011.01643.x. Br J Pharmacol. 2012. PMID: 21864314 Free PMC article. Review.
-
Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis.Curr Microbiol. 2009 Aug;59(2):118-22. doi: 10.1007/s00284-009-9408-9. Epub 2009 Apr 14. Curr Microbiol. 2009. PMID: 19365686
References
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev Microbiol. 2004;2:95–108. - PubMed
-
- Sutherland IW. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology. 2001;147:3–9. - PubMed
-
- Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–6. - PubMed
-
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. - PubMed
-
- Haesebrouck F, Chiers K, Van Oberbeke I, Ducatelle R. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol. 1997;58:239–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

