Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus
- PMID: 17412773
- PMCID: PMC2170854
- DOI: 10.1113/jphysiol.2006.127381
Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus
Abstract
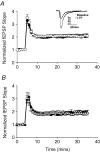
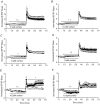
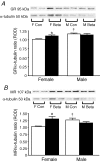
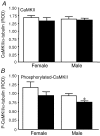
Synthetic glucocorticoids (sGCs) are routinely used to treat women at risk of preterm labour to promote fetal lung maturation. There is now strong evidence that exposure to excess glucocorticoid during periods of rapid brain development has permanent consequences for endocrine function and behaviour in the offspring. Prenatal exposure to sGC alters the expression of N-methyl-D-aspartate receptor (NMDA-R) subunits in the fetal and neonatal hippocampus. Given the integral role of the NMDA-R in synaptic plasticity, we hypothesized that prenatal sGC exposure will have effects on hippocampal long-term potentiation (LTP) after birth. Further, this may occur in either the presence or absence of elevated cortisol concentrations, in vitro. Pregnant guinea-pigs were injected with betamethasone (Beta, 1 mg kg(-1)) or vehicle on gestational days (gd) 40, 41, 50, 51, 60 and 61 (term approximately 70 days), a regimen comparable to that given to pregnant women. On postnatal day 21, LTP was examined at Schaffer collateral synapses in the CA1 region of hippocampal slices prepared from juvenile animals exposed to betamethasone or vehicle, in utero. Subsequently, the acute glucocorticoid receptor (GR)- and mineralocorticoid receptor (MR)-dependent effects of cortisol (0.1-10 microM; bath applied 30 min before LTP induction) were examined. There was no effect of prenatal sGC treatment on LTP under basal conditions. The application of 10 microM cortisol depressed excitatory synaptic transmission in all treatment groups regardless of sex. Similarly, LTP was depressed by 10 microM cortisol in all groups, with the exception of Beta-exposed females, in which LTP was unaltered. Hippocampal MR and GR protein levels were increased in Beta-exposed females, but not in any other prenatal treatment group. This study reveals sex-specific effects of prenatal exposure to sGC on LTP in the presence of elevated cortisol, a situation that would occur in vivo during stress.
Figures
Similar articles
-
Chronic prenatal ethanol exposure increases glucocorticoid-induced glutamate release in the hippocampus of the near-term foetal guinea pig.J Neuroendocrinol. 2006 Nov;18(11):826-34. doi: 10.1111/j.1365-2826.2006.01479.x. J Neuroendocrinol. 2006. PMID: 17026532
-
Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function in juvenile guinea pigs.J Neuroendocrinol. 2007 Mar;19(3):172-80. doi: 10.1111/j.1365-2826.2006.01517.x. J Neuroendocrinol. 2007. PMID: 17280590
-
Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs.J Physiol. 2007 Jan 1;578(Pt 1):249-57. doi: 10.1113/jphysiol.2006.122887. Epub 2006 Oct 26. J Physiol. 2007. PMID: 17068098 Free PMC article.
-
Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids.J Physiol. 2006 Apr 1;572(Pt 1):31-44. doi: 10.1113/jphysiol.2006.105254. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469780 Free PMC article. Review.
-
Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk.Endocr Rev. 2013 Dec;34(6):885-916. doi: 10.1210/er.2013-1012. Epub 2013 Aug 22. Endocr Rev. 2013. PMID: 23970762 Review.
Cited by
-
Gestational exposure to variable stressors produces decrements in cognitive and neural development of juvenile male and female rats.Curr Top Med Chem. 2011;11(13):1706-13. doi: 10.2174/156802611796117649. Curr Top Med Chem. 2011. PMID: 21463252 Free PMC article.
-
Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects.Endocrinology. 2012 Jul;153(7):3269-83. doi: 10.1210/en.2011-2160. Epub 2012 May 7. Endocrinology. 2012. PMID: 22564977 Free PMC article.
-
Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic.J Physiol. 2018 Dec;596(23):5535-5569. doi: 10.1113/JP274948. Epub 2018 May 30. J Physiol. 2018. PMID: 29633280 Free PMC article.
-
Neurobehavioral risk is associated with gestational exposure to stress hormones.Expert Rev Endocrinol Metab. 2012 Jul;7(4):445-459. doi: 10.1586/eem.12.33. Expert Rev Endocrinol Metab. 2012. PMID: 23144647 Free PMC article.
-
Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring.Schizophr Bull. 2009 May;35(3):509-27. doi: 10.1093/schbul/sbn176. Epub 2009 Jan 20. Schizophr Bull. 2009. PMID: 19155345 Free PMC article. Review.
References
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. - PubMed
-
- Beato M, Chavez S, Truss M. Transcriptional regulation by steroid hormones. Steroids. 1996;61:240–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

