Endothelial potential of human embryonic stem cells
- PMID: 17412888
- PMCID: PMC1924772
- DOI: 10.1182/blood-2006-08-019190
Endothelial potential of human embryonic stem cells
Abstract
Growing interest in using endothelial cells for therapeutic purposes has led to exploring human embryonic stem cells as a potential source for endothelial progenitor cells. Embryonic stem cells are advantageous when compared with other endothelial cell origins, due to their high proliferation capability, pluripotency, and low immunogenity. However, there are many challenges and obstacles to overcome before the vision of using embryonic endothelial progenitor cells in the clinic can be realized. Among these obstacles is the development of a productive method of isolating endothelial cells from human embryonic stem cells and elucidating their differentiation pathway. This review will focus on the endothelial potential of human embryonic stem cells that is described in current studies, with respect to the differentiation of human embryonic stem cells to endothelial cells, their isolation, and their characterization.
Figures

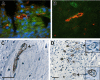


Similar articles
-
Human embryonic stem cells and liver diseases: from basic research to future clinical application.J Dig Dis. 2008 Feb;9(1):14-9. doi: 10.1111/j.1443-9573.2007.00319.x. J Dig Dis. 2008. PMID: 18251789 Review.
-
Human stem cells as a model for cardiac differentiation and disease.Cell Mol Life Sci. 2009 Mar;66(5):800-13. doi: 10.1007/s00018-009-8476-0. Cell Mol Life Sci. 2009. PMID: 19151924 Free PMC article. Review.
-
The cell cycle in stem cell proliferation, pluripotency and differentiation.Nat Cell Biol. 2019 Sep;21(9):1060-1067. doi: 10.1038/s41556-019-0384-4. Epub 2019 Sep 2. Nat Cell Biol. 2019. PMID: 31481793 Free PMC article. Review.
-
Role of SOX17 in hematopoietic development from human embryonic stem cells.Blood. 2013 Jan 17;121(3):447-58. doi: 10.1182/blood-2012-05-431403. Epub 2012 Nov 20. Blood. 2013. PMID: 23169777
-
Development of hematopoietic and endothelial cells from human embryonic stem cells: lessons from the studies using mouse as a model.ScientificWorldJournal. 2007 Dec 10;7:1950-64. doi: 10.1100/tsw.2007.310. ScientificWorldJournal. 2007. PMID: 18167610 Free PMC article. Review.
Cited by
-
Embryoid bodies as a model system for exploring early human embryonic development.J Assist Reprod Genet. 2025 Jun 17. doi: 10.1007/s10815-025-03546-x. Online ahead of print. J Assist Reprod Genet. 2025. PMID: 40526236 Review.
-
Efficient derivation of lateral plate and paraxial mesoderm subtypes from human embryonic stem cells through GSKi-mediated differentiation.Stem Cells Dev. 2013 Jul 1;22(13):1893-906. doi: 10.1089/scd.2012.0590. Epub 2013 Mar 28. Stem Cells Dev. 2013. PMID: 23413973 Free PMC article.
-
High-purity enrichment of functional cardiovascular cells from human iPS cells.Cardiovasc Res. 2012 Aug 1;95(3):327-35. doi: 10.1093/cvr/cvs185. Epub 2012 Jun 6. Cardiovasc Res. 2012. PMID: 22673369 Free PMC article.
-
A Scalable and Efficient Bioprocess for Manufacturing Human Pluripotent Stem Cell-Derived Endothelial Cells.Stem Cell Reports. 2018 Aug 14;11(2):454-469. doi: 10.1016/j.stemcr.2018.07.001. Epub 2018 Aug 2. Stem Cell Reports. 2018. PMID: 30078557 Free PMC article.
-
BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway.J Cell Biochem. 2010 Feb 1;109(2):363-74. doi: 10.1002/jcb.22410. J Cell Biochem. 2010. PMID: 19950207 Free PMC article.
References
-
- Liew A, Barry F, O'Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays. 2006;28:261–270. - PubMed
-
- Niklason LE. Techview: medical technology. Replacement arteries made to order. Science. 1999;286:1493–1494. - PubMed
-
- Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. - PubMed
-
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–199. - PubMed
-
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

