Genes required for mitotic spindle assembly in Drosophila S2 cells
- PMID: 17412918
- PMCID: PMC2837481
- DOI: 10.1126/science.1141314
Genes required for mitotic spindle assembly in Drosophila S2 cells
Abstract
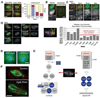
The formation of a metaphase spindle, a bipolar microtubule array with centrally aligned chromosomes, is a prerequisite for the faithful segregation of a cell's genetic material. Using a full-genome RNA interference screen of Drosophila S2 cells, we identified about 200 genes that contribute to spindle assembly, more than half of which were unexpected. The screen, in combination with a variety of secondary assays, led to new insights into how spindle microtubules are generated; how centrosomes are positioned; and how centrioles, centrosomes, and kinetochores are assembled.
Figures



References
-
- Mitchison TJ, Salmon ED. Nat. Cell Biol. 2001;3:E17. - PubMed
-
- McIntosh JR, Grishchuk EL, West RR. Annu. Rev. Cell Dev. Biol. 2002;18:193. - PubMed
-
- Rieder CL, Khodjakov A. Science. 2003;300:91. - PubMed
-
- Gadde S, Heald R. Curr. Biol. 2004;14:R797. - PubMed
-
- Scholey JM, Brust-Mascher I, Mogilner A. Nature. 2003;422:746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

