An ATP gate controls tubulin binding by the tethered head of kinesin-1
- PMID: 17412962
- PMCID: PMC2504013
- DOI: 10.1126/science.1136985
An ATP gate controls tubulin binding by the tethered head of kinesin-1
Abstract
Kinesin-1 is a two-headed molecular motor that walks along microtubules, with each step gated by adenosine triphosphate (ATP) binding. Existing models for the gating mechanism propose a role for the microtubule lattice. We show that unpolymerized tubulin binds to kinesin-1, causing tubulin-activated release of adenosine diphosphate (ADP). With no added nucleotide, each kinesin-1 dimer binds one tubulin heterodimer. In adenylyl-imidodiphosphate (AMP-PNP), a nonhydrolyzable ATP analog, each kinesin-1 dimer binds two tubulin heterodimers. The data reveal an ATP gate that operates independently of the microtubule lattice, by ATP-dependent release of a steric or allosteric block on the tubulin binding site of the tethered kinesin-ADP head.
Figures

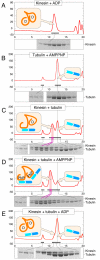


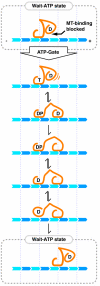
Comment in
-
Biochemistry. Processive motor movement.Science. 2007 Apr 6;316(5821):58-9. doi: 10.1126/science.1141549. Science. 2007. PMID: 17412943 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

