Ewing sarcoma gene EWS is essential for meiosis and B lymphocyte development
- PMID: 17415412
- PMCID: PMC1838927
- DOI: 10.1172/JCI31222
Ewing sarcoma gene EWS is essential for meiosis and B lymphocyte development
Abstract
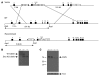
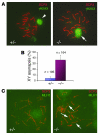
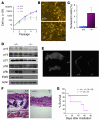
Ewing sarcoma gene EWS encodes a putative RNA-binding protein with proposed roles in transcription and splicing, but its physiological role in vivo remains undefined. Here, we have generated Ews-deficient mice and demonstrated that EWS is required for the completion of B cell development and meiosis. Analysis of Ews(-/-) lymphocytes revealed a cell-autonomous defect in precursor B lymphocyte (pre-B lymphocyte) development. During meiosis, Ews-null spermatocytes were deficient in XY bivalent formation and showed reduced meiotic recombination, resulting in massive apoptosis and complete arrest in gamete maturation. Inactivation of Ews in mouse embryonic fibroblasts resulted in premature cellular senescence, and the mutant animals showed hypersensitivity to ionizing radiation. Finally, we showed that EWS interacts with lamin A/C and that loss of EWS results in a reduced lamin A/C expression. Our findings reveal essential functions for EWS in pre-B cell development and meiosis, with proposed roles in DNA pairing and recombination/repair mechanisms. Furthermore, we demonstrate a novel role of EWS in cellular senescence, possibly through its interaction and modulation of lamin A/C.
Figures



References
-
- Delattre O., et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. - PubMed
-
- Arvand A., Denny C.T. Biology of EWS/ETS fusions in Ewing’s family tumors. Oncogene. 2001;20:5747–5754. - PubMed
-
- Janknecht R. EWS-ETS oncoproteins: the linchpins of Ewing tumors. Gene. 2005;363:1–14. - PubMed
-
- Zucman J., et al. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat. Genet. 1993;4:341–345. - PubMed
-
- Panagopoulos I., et al. Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene. 1996;12:489–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

