Apolipoprotein-J prevention of fetal cardiac myoblast apoptosis induced by ethanol
- PMID: 17416353
- PMCID: PMC3221769
- DOI: 10.1016/j.bbrc.2007.03.109
Apolipoprotein-J prevention of fetal cardiac myoblast apoptosis induced by ethanol
Abstract
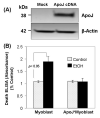
Over-consumption of ethanol (EtOH) represents a major health problem. This study was to test the cytotoxicity of EtOH in cardiac stem cells or myoblasts, and the potential protective effect of apolipoprotein-J (ApoJ), a stress-responding, chaperone-like protein in high-density lipoprotein, on EtOH-injured cardiac myoblasts. In culture, EtOH-exposed canine fetal myoblasts underwent apoptosis in a concentration- and time-dependent manner. Expression ApoJ by cDNA transfection markedly reduced EtOH-induced apoptosis in the cells. ApoJ expression also restored partially the mitochondrial membrane potential and prevented the release of cytochrome-c from mitochondria into cytoplasma. Thus, ApoJ serves as a cytoprotective protein that protects cardiac stem cells against EtOH cytotoxicity.
Figures



References
-
- Di Gennaro C, Biggi A, Barilli AL, Fasoli E, Carra N, Novarini A, Delsignore R, Montanari A. Endothelial dysfunction and cardiovascular risk profile in long-term withdrawing alcoholics. J Hypertens. 2007;25:367–73. - PubMed
-
- Rubert G, Minana R, Pascual M, Guerri C. Ethanol exposure during embryogenesis decreases the radial glial progenitorpool and affects the generation of neurons and astrocytes. J Neurosci Res. 2006;84:483–96. - PubMed
-
- Kang K, Oh YK, Choue R, Kang SJ. Scutellariae radix extracts suppress ethanol-induced caspase-11 expression and cell death in N(2)a cells. Brain Res Mol Brain Res. 2005;142:139–45. - PubMed
-
- Bhave SV, Hoffman PL. Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J Neurochem. 1997;68:578–86. - PubMed
-
- Li Y, King MA, Meyer EM. alpha7 nicotinic receptor-mediated protection against ethanol-induced oxidative stress and cytotoxicity in PC12 cells. Brain Res. 2000;861:165–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

