A freeze-fracture transmission electron microscopy and small angle x-ray diffraction study of the effects of albumin, serum, and polymers on clinical lung surfactant microstructure
- PMID: 17416614
- PMCID: PMC1914435
- DOI: 10.1529/biophysj.106.095513
A freeze-fracture transmission electron microscopy and small angle x-ray diffraction study of the effects of albumin, serum, and polymers on clinical lung surfactant microstructure
Abstract
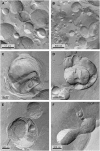
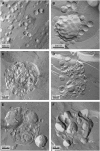
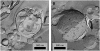
Freeze-fracture transmission electron microscopy shows significant differences in the bilayer organization and fraction of water within the bilayer aggregates of clinical lung surfactants, which increases from Survanta to Curosurf to Infasurf. Albumin and serum inactivate all three clinical surfactants in vitro; addition of the nonionic polymers polyethylene glycol, dextran, or hyaluronic acid also reduces inactivation in all three. Freeze-fracture transmission electron microscopy shows that polyethylene glycol, hyaluronic acid, and albumin do not adsorb to the surfactant aggregates, nor do these macromolecules penetrate the interior water compartments of the surfactant aggregates. This results in an osmotic pressure difference that dehydrates the bilayer aggregates, causing a decrease in the bilayer spacing as shown by small angle x-ray scattering and an increase in the ordering of the bilayers as shown by freeze-fracture electron microscopy. Small angle x-ray diffraction shows that the relationship between the bilayer spacing and the imposed osmotic pressure for Curosurf is a screened electrostatic interaction with a Debye length consistent with the ionic strength of the solution. The variation in surface tension due to surfactant adsorption measured by the pulsating bubble method shows that the extent of surfactant aggregate reorganization does not correlate with the maximum or minimum surface tension achieved with or without serum in the subphase. Albumin, polymers, and their mixtures alter the surfactant aggregate microstructure in the same manner; hence, neither inhibition reversal due to added polymer nor inactivation due to albumin is caused by alterations in surfactant microstructure.
Figures









References
-
- Clements, J. A., and M. E. Avery. 1998. Lung surfactant and neonatal respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 157:59–66. - PubMed
-
- Zasadzinski, J. A., J. Ding, H. E. Warriner, F. Bringezu, and A. J. Waring. 2001. The physics and physiology of lung surfactants. Curr. Opinion Coll. Int. Sci. 6:506–513.
-
- Avery, M. E. 2000. Surfactant deficiency in hyaline membrane disease. Am. J. Respir. Crit. Care Med. 161:1074–1075. - PubMed
-
- Notter, R. H. 2000. Lung Surfactant: Basic Science and Clinical Applications. Marcel Dekker, New York.
-
- Suresh, G. K., and R. F. Soll. 2005. Overview of surfactant replacement trials. J. Perinatol. 25:S40–S44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

