Regulation of arbuscular mycorrhization by carbon. The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity
- PMID: 17416641
- PMCID: PMC1851815
- DOI: 10.1104/pp.106.096446
Regulation of arbuscular mycorrhization by carbon. The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity
Erratum in
- Plant Physiol. 2007 Jun;144(2):1233
Abstract
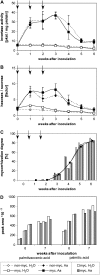
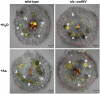
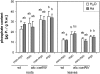
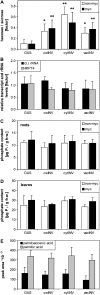
The mutualistic interaction in arbuscular mycorrhiza (AM) is characterized by an exchange of mineral nutrients and carbon. The major benefit of AM, which is the supply of phosphate to the plant, and the stimulation of mycorrhization by low phosphate fertilization has been well studied. However, less is known about the regulatory function of carbon availability on AM formation. Here the effect of enhanced levels of hexoses in the root, the main form of carbohydrate used by the fungus, on AM formation was analyzed. Modulation of the root carbohydrate status was performed by expressing genes encoding a yeast (Saccharomyces cerevisiae)-derived invertase, which was directed to different subcellular locations. Using tobacco (Nicotiana tabacum) alcc::wINV plants, the yeast invertase was induced in the whole root system or in root parts. Despite increased hexose levels in these roots, we did not detect any effect on the colonization with Glomus intraradices analyzed by assessment of fungal structures and the level of fungus-specific palmitvaccenic acid, indicative for the fungal carbon supply, or the plant phosphate content. Roots of Medicago truncatula, transformed to express genes encoding an apoplast-, cytosol-, or vacuolar-located yeast-derived invertase, had increased hexose-to-sucrose ratios compared to beta-glucuronidase-transformed roots. However, transformations with the invertase genes did not affect mycorrhization. These data suggest the carbohydrate supply in AM cannot be improved by root-specifically increased hexose levels, implying that under normal conditions sufficient carbon is available in mycorrhizal roots. In contrast, tobacco rolC::ppa plants with defective phloem loading and tobacco pyk10::InvInh plants with decreased acid invertase activity in roots exhibited a diminished mycorrhization.
Figures



Similar articles
-
Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction.Plant J. 2007 Aug;51(3):390-405. doi: 10.1111/j.1365-313X.2007.03150.x. Epub 2007 May 23. Plant J. 2007. PMID: 17521407
-
Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots.J Exp Bot. 2006;57(15):4015-23. doi: 10.1093/jxb/erl172. Epub 2006 Oct 18. J Exp Bot. 2006. PMID: 17050639
-
Constitutive overexpression of the sucrose transporter SoSUT1 in potato plants increases arbuscular mycorrhiza fungal root colonization under high, but not under low, soil phosphorus availability.J Plant Physiol. 2011 Jun 15;168(9):911-9. doi: 10.1016/j.jplph.2010.11.026. Epub 2011 Mar 5. J Plant Physiol. 2011. PMID: 21382646
-
Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter?Trends Plant Sci. 2017 Aug;22(8):652-660. doi: 10.1016/j.tplants.2017.05.008. Epub 2017 Jun 13. Trends Plant Sci. 2017. PMID: 28622919 Review.
-
Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review.Plant Biol (Stuttg). 2010 Mar;12(2):292-301. doi: 10.1111/j.1438-8677.2009.00312.x. Plant Biol (Stuttg). 2010. PMID: 20398236 Review.
Cited by
-
Apoplastic invertases: Multi-faced players in the arbuscular mycorrhization.Plant Signal Behav. 2008 May;3(5):317-9. doi: 10.4161/psb.3.5.5307. Plant Signal Behav. 2008. PMID: 19841657 Free PMC article.
-
A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants.Plant Cell. 2011 Oct;23(10):3812-23. doi: 10.1105/tpc.111.089813. Epub 2011 Oct 4. Plant Cell. 2011. PMID: 21972259 Free PMC article.
-
Laser microdissection unravels cell-type-specific transcription in arbuscular mycorrhizal roots, including CAAT-box transcription factor gene expression correlating with fungal contact and spread.Plant Physiol. 2011 Dec;157(4):2023-43. doi: 10.1104/pp.111.186635. Epub 2011 Oct 27. Plant Physiol. 2011. PMID: 22034628 Free PMC article.
-
Molecular Regulation of Arbuscular Mycorrhizal Symbiosis.Int J Mol Sci. 2022 May 25;23(11):5960. doi: 10.3390/ijms23115960. Int J Mol Sci. 2022. PMID: 35682640 Free PMC article. Review.
-
Bioactive molecules in soil ecosystems: masters of the underground.Int J Mol Sci. 2013 Apr 24;14(5):8841-68. doi: 10.3390/ijms14058841. Int J Mol Sci. 2013. PMID: 23615474 Free PMC article. Review.
References
-
- Blee KA, Anderson AJ (1998) Regulation of arbuscule formation by carbon in the plant. Plant J 16 523–530
-
- Blee KA, Anderson AJ (2002) Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Mol Biol 50 197–211 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

