Flagellar motility is critical for Listeria monocytogenes biofilm formation
- PMID: 17416647
- PMCID: PMC1913361
- DOI: 10.1128/JB.01967-06
Flagellar motility is critical for Listeria monocytogenes biofilm formation
Abstract
The food-borne pathogen Listeria monocytogenes attaches to environmental surfaces and forms biofilms that can be a source of food contamination, yet little is known about the molecular mechanisms of its biofilm development. We observed that nonmotile mutants were defective in biofilm formation. To investigate how flagella might function during biofilm formation, we compared the wild type with flagellum-minus and paralyzed-flagellum mutants. Both nonmotile mutants were defective in biofilm development, presumably at an early stage, as they were also defective in attachment to glass during the first few hours of surface exposure. This attachment defect could be significantly overcome by providing exogenous movement toward the surface via centrifugation. However, this centrifugation did not restore mature biofilm formation. Our results indicate that it is flagellum-mediated motility that is critical for both initial surface attachment and subsequent biofilm formation. Also, any role for L. monocytogenes flagella as adhesins on abiotic surfaces appears to be either minimal or motility dependent under the conditions we examined.
Figures
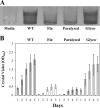
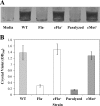
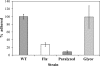
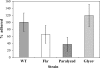
References
-
- Ayebah, B., Y. C. Hung, and J. F. Frank. 2005. Enhancing the bactericidal effect of electrolyzed water on Listeria monocytogenes biofilms formed on stainless steel. J. Food Prot. 68:1375-1380. - PubMed
-
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

