The phosphotransferase system formed by PtsP, PtsO, and PtsN proteins controls production of polyhydroxyalkanoates in Pseudomonas putida
- PMID: 17416664
- PMCID: PMC1913348
- DOI: 10.1128/JB.00033-07
The phosphotransferase system formed by PtsP, PtsO, and PtsN proteins controls production of polyhydroxyalkanoates in Pseudomonas putida
Abstract
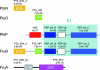
The genome of Pseudomonas putida KT2440 encodes five proteins of the phosphoenolpyruvate-carbohydrate phosphotransferase system. Two of these (FruA and FruB) form a dedicated system for fructose intake, while enzyme I(Ntr) (EI(Ntr); encoded by ptsP), NPr (ptsO), and EII(Ntr) (ptsN) act in concert to control the intracellular accumulation of polyhydroxyalkanoates, a typical product of carbon overflow.
Figures



Similar articles
-
Evidence of in vivo cross talk between the nitrogen-related and fructose-related branches of the carbohydrate phosphotransferase system of Pseudomonas putida.J Bacteriol. 2008 May;190(9):3374-80. doi: 10.1128/JB.02002-07. Epub 2008 Feb 22. J Bacteriol. 2008. PMID: 18296519 Free PMC article.
-
The interplay of the EIIA(Ntr) component of the nitrogen-related phosphotransferase system (PTS(Ntr)) of Pseudomonas putida with pyruvate dehydrogenase.Biochim Biophys Acta. 2011 Oct;1810(10):995-1005. doi: 10.1016/j.bbagen.2011.01.002. Epub 2011 Jan 12. Biochim Biophys Acta. 2011. PMID: 21236318
-
Enzyme I NPr, NPr and IIA Ntr are involved in regulation of the poly-beta-hydroxybutyrate biosynthetic genes in Azotobacter vinelandii.J Mol Microbiol Biotechnol. 2008;15(4):244-54. doi: 10.1159/000108658. Epub 2007 Sep 20. J Mol Microbiol Biotechnol. 2008. PMID: 17878711
-
From the phosphoenolpyruvate phosphotransferase system to selfish metabolism: a story retraced in Pseudomonas putida.FEMS Microbiol Lett. 2014 Jul;356(2):144-53. doi: 10.1111/1574-6968.12459. Epub 2014 May 29. FEMS Microbiol Lett. 2014. PMID: 24801646 Review.
-
The complete phosphotransferase system in Escherichia coli.J Mol Microbiol Biotechnol. 2001 Jul;3(3):329-46. J Mol Microbiol Biotechnol. 2001. PMID: 11361063 Review.
Cited by
-
Unnecessary signaling: poorly named?J Bacteriol. 2011 Sep;193(18):4571-3. doi: 10.1128/JB.05682-11. Epub 2011 Jul 8. J Bacteriol. 2011. PMID: 21742895 Free PMC article. No abstract available.
-
The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways.J Bacteriol. 2010 Jun;192(12):3055-67. doi: 10.1128/JB.00213-10. Epub 2010 Apr 16. J Bacteriol. 2010. PMID: 20400550 Free PMC article.
-
Implications of various phosphoenolpyruvate-carbohydrate phosphotransferase system mutations on glycerol utilization and poly(3-hydroxybutyrate) accumulation in Ralstonia eutropha H16.AMB Express. 2011 Jul 13;1(1):16. doi: 10.1186/2191-0855-1-16. AMB Express. 2011. PMID: 21906371 Free PMC article.
-
Effects of homologous phosphoenolpyruvate-carbohydrate phosphotransferase system proteins on carbohydrate uptake and poly(3-Hydroxybutyrate) accumulation in Ralstonia eutropha H16.Appl Environ Microbiol. 2011 Jun;77(11):3582-90. doi: 10.1128/AEM.00218-11. Epub 2011 Apr 8. Appl Environ Microbiol. 2011. PMID: 21478317 Free PMC article.
-
Transcriptional Study of the RsmZ-sRNAs and Their Relationship to the Biosynthesis of Alginate and Alkylresorcinols in Azotobacter vinelandii.Mol Biotechnol. 2018 Sep;60(9):670-680. doi: 10.1007/s12033-018-0102-7. Mol Biotechnol. 2018. PMID: 29987520
References
-
- Bordo, D., R. L. van Monfort, T. Pijning, K. H. Kalk, J. Reizer, M. H. Saier, Jr., and B. W. Dijkstra. 1998. The three-dimensional structure of the nitrogen regulatory protein IIANtr from Escherichia coli. J. Mol. Biol. 279:245-255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

