Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance
- PMID: 17416680
- PMCID: PMC1885613
- DOI: 10.1073/pnas.0608703104
Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance
Abstract
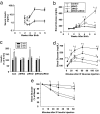
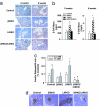
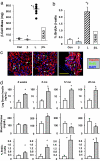
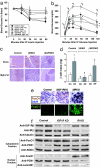
Insulin and insulin-like growth factor 1 (IGF1) are ubiquitous growth factors that regulate proliferation in most mammalian tissues including pancreatic islets. To explore the specificity of insulin receptors in compensatory beta-cell growth, we examined two models of insulin resistance. In the first model, we used liver-specific insulin receptor knockout (LIRKO) mice, which exhibit hyperinsulinemia without developing diabetes due to a compensatory increase in beta-cell mass. LIRKO mice, also lacking functional insulin receptors in beta-cells (beta IRKO/LIRKO), exhibited severe glucose intolerance but failed to develop compensatory islet hyperplasia, together leading to early death. In the second model, we examined the relative significance of insulin versus IGF1 receptors in islet growth by feeding high-fat diets to beta IRKO and beta-cell-specific IGF1 receptor knockout (beta IGFRKO) mice. Although both groups on the high-fat diet developed insulin resistance, beta IRKO, but not beta IGFRKO, mice exhibited poor islet growth consistent with insulin-stimulated phosphorylation, nuclear exclusion of FoxO1, and reduced expression of Pdx-1. Together these data provide direct genetic evidence that insulin/FoxO1/Pdx-1 signaling is one pathway that is crucial for islet compensatory growth response to insulin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
When the usual insulin is just not enough.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8681-2. doi: 10.1073/pnas.0702844104. Epub 2007 May 16. Proc Natl Acad Sci U S A. 2007. PMID: 17517662 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

