Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: lack of a plastidic desaturation pathway results in the coupled pathway of galactolipid synthesis
- PMID: 17416897
- PMCID: PMC1951526
- DOI: 10.1128/EC.00393-06
Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: lack of a plastidic desaturation pathway results in the coupled pathway of galactolipid synthesis
Abstract
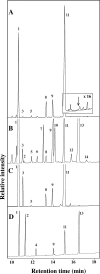
The acyl lipids making up the plastid membranes in plants and algae are highly enriched in polyunsaturated fatty acids and are synthesized by two distinct pathways, known as the prokaryotic and eukaryotic pathways, which are located within the plastids and the endoplasmic reticulum, respectively. Here we report the results of biochemical as well as genomic analyses of lipids and fatty acids in the unicellular rhodophyte Cyanidioschyzon merolae. All of the glycerolipids usually found in photosynthetic algae were found, such as mono- and digalactosyl diacylglycerol, sulfolipid, phosphatidylglycerol, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol. However, the fatty acid composition was extremely simple. Only palmitic, stearic, oleic, and linoleic acids were found as major acids. In addition, 3-trans-hexadecanoic acid was found as a very minor component in phosphatidylglycerol. Unlike the case for most other photosynthetic eukaryotes, polyenoic fatty acids having three or more double bonds were not detected. These results suggest that polyunsaturated fatty acids are not necessary for photosynthesis in eukaryotes. Genomic analysis suggested that C. merolae lacks acyl lipid desaturases of cyanobacterial origin as well as stearoyl acyl carrier protein desaturase, both of which are major desaturases in plants and green algae. The results of labeling experiments with radioactive acetate showed that the desaturation leading to linoleic acid synthesis occurs on phosphatidylcholine located outside the plastids. Monogalactosyl diacylglycerol is therefore synthesized by the coupled pathway, using plastid-derived palmitic acid and endoplasmic reticulum-derived linoleic acid. These results highlight essential differences in lipid biosynthetic pathways between the red algae and the green lineage, which includes plants and green algae.
Figures






References
-
- Adachi, J., and M. Hasegawa. 1992. Amino acid substitution of proteins coded for in mitochondrial DNA during mammalian evolution. Jpn. J. Genet. 67:187-197. - PubMed
-
- Allen, M. B. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Microbiol. 32:270-277. - PubMed
-
- Araki, S., T. Sakurai, T. Omata, A. Kawaguchi, and N. Murata. 1986. Lipid and fatty acid composition in the red alga Porphyra yezoensis. Jpn. J. Phycol. 34:94-100.
-
- Armbrust, E. V., J. A. Berges, C. Bowler, B. R. Green, D. Martinez, N. H. Putnam, S. Zhou, A. E. Allen, K. E. Apt, M. Bechner, M. A. Brzezinski, B. K. Chaal, A. Chiovitti, A. K. Davis, M. S. Demarest, J. C. Detter, T. Glavina, D. Goodstein, M. Z. Hadi, U. Hellsten, M. Hildebrand, B. D. Jenkins, J. Jurka, V. V. Kapitonov, N. Kroger, W. W. Lau, T. W. Lane, F. W. Larimer, J. C. Lippmeier, S. Lucas, M. Medina, A. Montsant, M. Obornik, M. S. Parker, B. Palenik, G. J. Pazour, P. M. Richardson, T. A. Rynearson, M. A. Saito, D. C. Schwartz, K. Thamatrakoln, K. Valentin, A. Vardi, F. P. Wilkerson, and D. S. Rokhsar. 2004. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

