Membrane topography of the hydrophobic anchor sequence of poliovirus 3A and 3AB proteins and the functional effect of 3A/3AB membrane association upon RNA replication
- PMID: 17417822
- PMCID: PMC2519882
- DOI: 10.1021/bi6024758
Membrane topography of the hydrophobic anchor sequence of poliovirus 3A and 3AB proteins and the functional effect of 3A/3AB membrane association upon RNA replication
Abstract
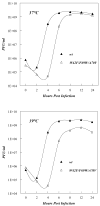
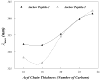
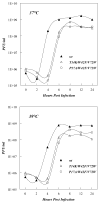
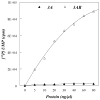
Replication of poliovirus RNA takes place on the cytoplasmic surface of membranous vesicles that form after infection of the host cell. It is generally accepted that RNA polymerase 3D(pol) interacts with membranes in a complex with viral protein 3AB, which binds to membranes by means of a hydrophobic anchor sequence that is located near the C-terminus of the 3A domain. In this study, we used fluorescence and fluorescence quenching methods to define the topography of the anchor sequence in the context of 3A and 3AB proteins inserted in model membranes. Mutants with a single tryptophan near the center of the anchor sequence but lacking Trp elsewhere in 3A/3AB were constructed which, after the emergence of suppressor mutations, replicated well in HeLa cells. When a peptide containing the mutant anchor sequence was incorporated in model membrane vesicles, measurements of Trp depth within the lipid bilayer indicated formation of a transmembrane topography. However, rather than the 22-residue length predicted from hydrophobicity considerations, the transmembrane segment had an effective length of 16 residues, such that Gln64 likely formed the N-terminal boundary. Analogous experiments using full-length proteins bound to preformed model membrane vesicles showed that the anchor sequence formed a mixture of transmembrane and nontransmembrane topographies in the 3A protein but adopted only the nontransmembrane configuration in the context of 3AB protein. Studies of the function of 3A/3AB inserted into model membrane vesicles showed that membrane-bound 3AB is highly efficient in stimulating the activity of 3D(pol) in vitro while membrane-bound 3A totally lacks this activity. Moreover, in vitro uridylylation reactions showed that membrane-bound 3AB is not a substrate for 3D(pol), but free VPg released by cleavage of 3AB with proteinase 3CD(pro) could be uridylylated.
Figures

References
-
- Paul AV. Possible unifying mechanism of picornavirus genome replication. In: Semler BL, Wimmer E, editors. In molecular Biology of picornaviruses. ASM Press; Washington, DC: 2002. pp. 227–246.
-
- Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. - PubMed
-
- Egger D, Gosert R, Bienz K. Role of cellular structures in viral RNA replication. In: Semler BL, Wimmer E, editors. In Molecular Biology of Piocrnaviruses. ASM Press; Washington DC: 2002. pp. 20036–22904.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

