Efficient production and secretion of bovine beta-lactoglobulin by Lactobacillus casei
- PMID: 17417967
- PMCID: PMC1853110
- DOI: 10.1186/1475-2859-6-12
Efficient production and secretion of bovine beta-lactoglobulin by Lactobacillus casei
Abstract
Background: Lactic acid bacteria (LAB) are attractive tools to deliver therapeutic molecules at the mucosal level. The model LAB Lactococcus lactis has been intensively used to produce and deliver such heterologous proteins. However, compared to recombinant lactococci, lactobacilli offer some advantages such as better survival in the digestive tract and immunomodulatory properties. Here, we compared different strategies to optimize the production of bovine beta-lactoglobulin (BLG), a major cow's milk allergen, in the probiotic strain Lactobacillus casei BL23.
Results: Using a nisin-inducible plasmid system, we first showed that L. casei BL23 strain could efficiently secrete a reporter protein, the staphylococcal nuclease (Nuc), with the lactococcal signal peptide SPUsp45 fused to its N-terminus. The fusion of SPUsp45 failed to drive BLG secretion but led to a 10-fold increase of intracellular BLG production. Secretion was significantly improved when the synthetic propeptide LEISSTCDA (hereafter called LEISS) was added to the N-terminus of the mature moiety of BLG. Secretion rate of LEISS-BLG was 6-fold higher than that of BLG alone while intracellular production reached then about 1 mg/L of culture. The highest yield of secretion was obtained by using Nuc as carrier protein. Insertion of Nuc between LEISS and BLG resulted in a 20-fold increase in BLG secretion, up to 27 microg/L of culture. Furthermore, the lactococcal nisRK regulatory genes were integrated into the BL23 chromosome. The nisRK insertion allowed a decrease of BLG synthesis in uninduced cultures while BLG production increased by 50% after nisin induction. Moreover, modification of the induction protocol led to increase the proportion of soluble BLG to around 74% of the total BLG production.
Conclusion: BLG production and secretion in L. casei were significantly improved by fusions to a propeptide enhancer and a carrier protein. The resulting recombinant strains will be further tested for their ability to modulate the immune response against BLG via mucosal delivery in a cow's milk allergy model in mice.
Figures

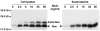

Similar articles
-
Improvement of bovine beta-lactoglobulin production and secretion by Lactococcus lactis.Braz J Med Biol Res. 2005 Mar;38(3):353-9. doi: 10.1590/s0100-879x2005000300005. Epub 2005 Mar 8. Braz J Med Biol Res. 2005. PMID: 15761614
-
Constitutive delivery of bovine beta-lactoglobulin to the digestive tracts of gnotobiotic mice by engineered Lactobacillus casei.Appl Environ Microbiol. 2006 Dec;72(12):7460-7. doi: 10.1128/AEM.01032-06. Epub 2006 Sep 22. Appl Environ Microbiol. 2006. PMID: 16997983 Free PMC article.
-
Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin.Clin Diagn Lab Immunol. 2001 May;8(3):545-51. doi: 10.1128/CDLI.8.3.545-551.2001. Clin Diagn Lab Immunol. 2001. PMID: 11329455 Free PMC article.
-
Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system.Braz J Med Biol Res. 1999 Feb;32(2):191-8. doi: 10.1590/s0100-879x1999000200007. Braz J Med Biol Res. 1999. PMID: 10347754 Review.
-
Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines.Microb Cell Fact. 2011 Aug 30;10 Suppl 1(Suppl 1):S4. doi: 10.1186/1475-2859-10-S1-S4. Epub 2011 Aug 30. Microb Cell Fact. 2011. PMID: 21995317 Free PMC article. Review.
Cited by
-
K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice.Vaccines (Basel). 2021 Feb 26;9(3):195. doi: 10.3390/vaccines9030195. Vaccines (Basel). 2021. PMID: 33652552 Free PMC article.
-
Heterologous protein display on the cell surface of lactic acid bacteria mediated by the s-layer protein.Microb Cell Fact. 2011 Oct 28;10:86. doi: 10.1186/1475-2859-10-86. Microb Cell Fact. 2011. PMID: 22035337 Free PMC article.
-
Gut remediation: a potential approach to reducing chromium accumulation using Lactobacillus plantarum TW1-1.Sci Rep. 2017 Nov 8;7(1):15000. doi: 10.1038/s41598-017-15216-9. Sci Rep. 2017. PMID: 29118411 Free PMC article.
-
Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes.Diabetes. 2015 May;64(5):1794-803. doi: 10.2337/db14-0635. Epub 2015 Jan 27. Diabetes. 2015. PMID: 25626737 Free PMC article.
-
High yield recombinant thermostable alpha-amylase production using an improved Bacillus licheniformis system.Microb Cell Fact. 2009 Oct 31;8:58. doi: 10.1186/1475-2859-8-58. Microb Cell Fact. 2009. PMID: 19878591 Free PMC article.
References
-
- Rautava S, Kalliomaki M, Isolauri E. New therapeutic strategy for combating the increasing burden of allergic disease: Probiotics-A Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota (NAMI) Research Group report. J Allergy Clin Immunol. 2005;116:31–37. doi: 10.1016/j.jaci.2005.02.010. - DOI - PubMed
-
- Salminen S, Isolauri E. Intestinal colonization, microbiota, and probiotics. J Pediatr. 2006;149:S115–S120. doi: 10.1016/j.jpeds.2006.06.062. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

