Assembly of recently translated full-length and C-terminal truncated human gamma-globin chains with a pool of alpha-globin chains to form Hb F in a cell-free system
- PMID: 17418086
- PMCID: PMC1978184
- DOI: 10.1016/j.abb.2007.02.030
Assembly of recently translated full-length and C-terminal truncated human gamma-globin chains with a pool of alpha-globin chains to form Hb F in a cell-free system
Abstract
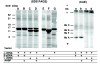
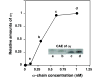
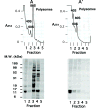
Assembly of alpha-globin with translated, full-length and C-terminal truncated human gamma-globin to form Hb F was assessed in a cell-free transcription/translation system. Polysome profiles showed two amino acid C-terminal-truncated gamma-chains retained on polysomes can assemble with unlabeled holo alpha-chains only after puromycin-induced chain release. Two amino acid C-terminal truncated gamma-chains encoded from vectors containing a stop codon at the translation termination site were released from polysomes and assembled with alpha-chains in the absence of puromycin addition, while removal of 11 or more amino acids from the gamma-chain carboxy-terminus inhibited assembly with alpha-chains. These results suggest that amino acids in the HC- and H-helix gamma-chain regions including amino acids 135-144 at the C-terminus in the translated gamma-chains play a key role in assembly with alpha-chains, and that assembly occurs soon after exit of translated gamma-chains from the ribosome tunnel and release from polysomes thereby preventing stable gamma(2) homo-dimer formation.
Figures

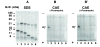
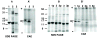
Similar articles
-
Assembly of human hemoglobin (Hb) beta- and gamma-globin chains expressed in a cell-free system with alpha-globin chains to form Hb A and Hb F.J Biol Chem. 2002 Apr 19;277(16):13415-20. doi: 10.1074/jbc.M200857200. Epub 2002 Feb 4. J Biol Chem. 2002. PMID: 11827978
-
Assembly of gamma- with alpha-globin chains to form human fetal hemoglobin in vitro and in vivo.J Biol Chem. 2000 Apr 28;275(17):12424-9. doi: 10.1074/jbc.c000137200. J Biol Chem. 2000. PMID: 10777526
-
Significance of beta116 His (G18) at alpha1beta1 contact sites for alphabeta assembly and autoxidation of hemoglobin.Biochemistry. 2003 Sep 2;42(34):10252-9. doi: 10.1021/bi030095s. Biochemistry. 2003. PMID: 12939154
-
Disorders of the synthesis of human fetal hemoglobin.IUBMB Life. 2008 Feb;60(2):94-111. doi: 10.1002/iub.4. IUBMB Life. 2008. PMID: 18379999 Review.
-
Understanding mechanisms of gamma-globin gene regulation to develop strategies for pharmacological fetal hemoglobin induction.Dev Dyn. 2006 Jul;235(7):1727-37. doi: 10.1002/dvdy.20802. Dev Dyn. 2006. PMID: 16607652 Review.
References
-
- Bunn FH. Blood. 1987;69:1–6. - PubMed
-
- Shaeffer JR, Kingston RE, McDonald MJ, Bunn HF. Nature. 1978;276:631–632. - PubMed
-
- Adachi K, Zhao Y, Yamaguchi T, Surrey S. J Biol Chem. 2000;275:12424–12429. - PubMed
-
- Adachi K, Zhao Y, Surrey S. J Biol Chem. 2002;277:13415–13420. - PubMed
-
- Stalling M, Abraham A, Abraham EC. Blood. 1983;62:75a. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

