The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis
- PMID: 17418411
- PMCID: PMC1885943
- DOI: 10.1016/j.ccr.2007.02.015
The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis
Abstract
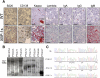
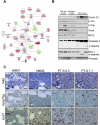
Multiple myeloma (MM) evolves from a highly prevalent premalignant condition termed MGUS. The factors underlying the malignant transformation of MGUS are unknown. We report a MGUS/MM phenotype in transgenic mice with Emu-directed expression of the XBP-1 spliced isoform (XBP-1s), a factor governing unfolded protein/ER stress response and plasma-cell development. Emu-XBP-1s elicited elevated serum Ig and skin alterations. With age, Emu-xbp-1s transgenics develop features diagnostic of human MM, including bone lytic lesions and subendothelial Ig deposition. Furthermore, transcriptional profiles of Emu-xbp-1s lymphoid and MM cells show aberrant expression of known human MM dysregulated genes. The similarities of this model with the human disease, coupled with documented frequent XBP-1s overexpression in human MM, serve to implicate XBP-1s dysregulation in MM pathogenesis.
Figures




References
-
- Bergsagel P.L., Kuehl W.M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 2005;23:6333–6338. - PubMed
-
- Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P., Clark S.G., Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
-
- Calvano S.E., Xiao W., Richards D.R., Felciano R.M., Baker H.V., Cho R.J., Chen R.O., Brownstein B.H., Cobb J.P., Tschoeke S.K. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. - PubMed
-
- Carrasco D., Tonon G., Huang Y., Zhang Y., Sinha R., Feng B., Zhan F., Khatry D., Protopopova M., Protopopov A. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

