Functional and molecular characterization of multiple K-Cl cotransporter isoforms in corneal epithelial cells
- PMID: 17418819
- PMCID: PMC2696115
- DOI: 10.1016/j.exer.2007.02.007
Functional and molecular characterization of multiple K-Cl cotransporter isoforms in corneal epithelial cells
Abstract
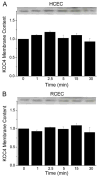
The dependence of regulatory volume decrease (RVD) activity on potassium-chloride cotransporter (KCC) isoform expression was characterized in corneal epithelial cells (CEC). During exposure to a 50% hypotonic challenge, the RVD response was larger in SV40-immortalized human CEC (HCEC) than in SV40-immortalized rabbit CEC (RCEC). A KCC inhibitor-[(dihydroindenyl)oxy] alkanoic acid (DIOA)-blocked RVD more in HCEC than RCEC. Under isotonic conditions, N-ethylmaleimide (NEM) produced KCC activation and transient cell shrinkage. Both of these changes were greater in HCEC than in RCEC. Immunoblot analysis of HCEC, RCEC, primary human CEC (pHCEC), and primary bovine CEC (BCEC) plasma membrane enriched fractions revealed KCC1, KCC3, and KCC4 isoform expression, whereas KCC2 was undetectable. During a hypotonic challenge, KCC1 membrane content increased more rapidly in HCEC than in RCEC. Such a challenge induced a larger increase and more transient p44/42MAPK activation in HCEC than RCEC. On the other hand, HCEC and RCEC p38MAPK phosphorylation reached peak activations at 2.5 and 15 min, respectively. Only in HCEC, pharmacological manipulation of KCC activity modified the hypotonicity-induced activation of p44/42MAPK, whereas p38MAPK phosphorylation was insensitive to such procedures in both cell lines. Larger increases in HCEC KCC1 membrane protein content correlated with their ability to undergo faster and more complete RVD. Furthermore, pharmacological activation of KCC increased p44/42MAPK phosphorylation in HCEC but not in RCEC, presumably a reflection of low KCC1 membrane expression in RCEC. These findings suggest that KCC1 plays a role in (i) maintaining isotonic steady-state cell volume homeostasis, (ii) recovery of isotonic cell volume after a hypotonic challenge through RVD, and (iii) regulating hypotonicity-induced activation of the p44/42MAPK signaling pathway required for cell proliferation.
Figures











References
-
- Adragna NC, Ferrell CM, Zhang J, Di Fulvio M, Temprana CF, Sharma A, Fyffe RE, Cool DR, Lauf PK. Signal transduction mechanisms of K+-Cl− cotransport regulation and relationship to disease. Acta Physiol (Oxf) 2006;187:125–139. - PubMed
-
- Adragna NC, Fulvio MD, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. - PubMed
-
- Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. J Membr Biol. 2004;201:41–49. - PubMed
-
- Al-Nakkash L, Reinach PS. Activation of a CFTR-mediated chloride current in a rabbit corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2001;42:2364–2370. - PubMed
-
- Araki K, Ohashi Y, Sasabe T, Kinoshita S, Hayashi K, Yang XZ, Hosaka Y, Aizawa S, Handa H. Immortalization of rabbit corneal epithelial cells by a recombinant SV40-adenovirus vector. Invest Ophthalmol Vis Sci. 1993;34:2665–2671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

