Anterograde tracing method using DiI to label vagal innervation of the embryonic and early postnatal mouse gastrointestinal tract
- PMID: 17418900
- PMCID: PMC1974840
- DOI: 10.1016/j.jneumeth.2007.03.001
Anterograde tracing method using DiI to label vagal innervation of the embryonic and early postnatal mouse gastrointestinal tract
Abstract
The mouse is an extremely valuable model for studying vagal development in relation to strain differences, genetic variation, gene manipulations or pharmacological manipulations. Therefore, a method using 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) was developed for labeling vagal innervation of the gastrointestinal (GI) tract in embryonic and postnatal mice. DiI labeling was adapted and optimized for this purpose by varying several facets of the method. For example, insertion and crushing of DiI crystals into the nerve led to faster DiI diffusion along vagal axons and diffusion over longer distances as compared with piercing the nerve with a micropipette tip coated with dried DiI oil. Moreover, inclusion of EDTA in the fixative reduced leakage of DiI out of nerve fibers that occurred with long incubations. Also, mounting labeled tissue in PBS was superior to glycerol with n-propyl gallate, which resulted in reduced clarity of DiI labeling that may have been due to DiI leaking out of fibers. Optical sectioning of flattened wholemounts permitted examination of individual tissue layers of the GI tract wall. This procedure aided identification of nerve ending types because in most instances each type innervates a different tissue layer. Between embryonic day 12.5 and postnatal day 8, growth of axons into the GI tract, formation and patterning of fiber bundles in the myenteric plexus and early formation of putative afferent and efferent nerve terminals were observed. Thus, the DiI tracing method developed here has opened up a window for investigation during an important phase of vagal development.
Figures
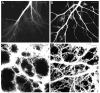

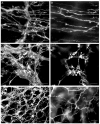
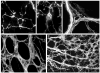
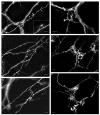

Similar articles
-
Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation.J Comp Neurol. 2010 Aug 1;518(15):2934-51. doi: 10.1002/cne.22372. J Comp Neurol. 2010. PMID: 20533354 Free PMC article.
-
Effect of temperature and calcium on transneuronal diffusion of DiI in fixed brain preparations.J Neurosci Methods. 1999 Apr 1;88(1):27-31. doi: 10.1016/s0165-0270(99)00007-2. J Neurosci Methods. 1999. PMID: 10379576
-
Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat.J Comp Neurol. 1990 Nov 1;301(1):65-79. doi: 10.1002/cne.903010107. J Comp Neurol. 1990. PMID: 1706359
-
Development of the intrinsic and extrinsic innervation of the gut.Dev Biol. 2016 Sep 15;417(2):158-67. doi: 10.1016/j.ydbio.2016.04.016. Epub 2016 Apr 22. Dev Biol. 2016. PMID: 27112528 Review.
-
A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake.Auton Neurosci. 2006 Jun 30;126-127:9-29. doi: 10.1016/j.autneu.2006.03.005. Epub 2006 May 4. Auton Neurosci. 2006. PMID: 16677865 Review.
Cited by
-
Mechanism of hyperphagia contributing to obesity in brain-derived neurotrophic factor knockout mice.Neuroscience. 2013 Jan 15;229:176-99. doi: 10.1016/j.neuroscience.2012.09.078. Epub 2012 Oct 13. Neuroscience. 2013. PMID: 23069761 Free PMC article.
-
Histological methods for ex vivo axon tracing: A systematic review.Neurol Res. 2016 Jul;38(7):561-9. doi: 10.1080/01616412.2016.1153820. Epub 2016 Apr 21. Neurol Res. 2016. PMID: 27098542 Free PMC article.
-
Mapping of Extrinsic Innervation of the Gastrointestinal Tract in the Mouse Embryo.J Neurosci. 2020 Aug 26;40(35):6691-6708. doi: 10.1523/JNEUROSCI.0309-20.2020. Epub 2020 Jul 20. J Neurosci. 2020. PMID: 32690615 Free PMC article.
-
Early stage transplantation of bone marrow cells markedly ameliorates copper metabolism and restores liver function in a mouse model of Wilson disease.BMC Gastroenterol. 2011 Jun 15;11:75. doi: 10.1186/1471-230X-11-75. BMC Gastroenterol. 2011. PMID: 21676234 Free PMC article.
-
Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation.J Comp Neurol. 2010 Aug 1;518(15):2934-51. doi: 10.1002/cne.22372. J Comp Neurol. 2010. PMID: 20533354 Free PMC article.
References
-
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–7. - PubMed
-
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol. 1997;195:183–91. - PubMed
-
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–76. - PubMed
-
- Boekelaar AB, Bloot J. Some aspects of the development of the peripheral autonomic nervous system in the abdomen and the pelvis of the rat Preliminary results. Acta Histochem Suppl. 1986;32:77–81. - PubMed
-
- Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

