Radiolabeled phenethylguanidines: novel imaging agents for cardiac sympathetic neurons and adrenergic tumors
- PMID: 17419605
- PMCID: PMC2625310
- DOI: 10.1021/jm061398y
Radiolabeled phenethylguanidines: novel imaging agents for cardiac sympathetic neurons and adrenergic tumors
Abstract
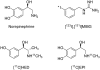
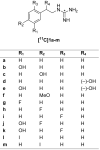
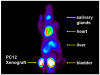
The norepinephrine transporter (NET) substrates [123I]-m-iodobenzylguanidine (MIBG) and [11C]-m-hydroxyephedrine (HED) are used as markers of cardiac sympathetic neurons and adrenergic tumors (pheochromocytoma, neuroblastoma). However, their rapid NET transport rates limit their ability to provide accurate measurements of cardiac nerve density. [11C]Phenethylguanidine ([11C]1a) and 12 analogues ([11C]1b-m) were synthesized and evaluated as radiotracers with improved kinetics for quantifying cardiac nerve density. In isolated rat hearts, neuronal uptake rates of [11C]1a-m ranged from 0.24 to 1.96 mL min-1 (g wet wt)-1, and six compounds had extremely long neuronal retention times (clearance T1/2 > 20 h) due to efficient vesicular storage. Positron emission tomography (PET) studies in nonhuman primates with [11C]1e, N-[11C]guanyl-m-octopamine, which has a slow NET transport rate, showed improved myocardial kinetics compared to HED. Compound [11C]1c, [11C]-p-hydroxyphenethylguanidine, which has a rapid NET transport rate, avidly accumulated into rat pheochromocytoma xenograft tumors in mice. These encouraging findings demonstrate that radiolabeled phenethylguanidines deserve further investigation as radiotracers of cardiac sympathetic innervation and adrenergic tumors.
Figures






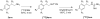
Similar articles
-
[18F]Fluoro-Hydroxyphenethylguanidines: Efficient Synthesis and Comparison of Two Structural Isomers as Radiotracers of Cardiac Sympathetic Innervation.ACS Chem Neurosci. 2017 Jul 19;8(7):1530-1542. doi: 10.1021/acschemneuro.7b00051. Epub 2017 Mar 27. ACS Chem Neurosci. 2017. PMID: 28322043 Free PMC article.
-
Quantification of cardiac sympathetic nerve density with N-11C-guanyl-meta-octopamine and tracer kinetic analysis.J Nucl Med. 2013 Sep;54(9):1645-52. doi: 10.2967/jnumed.113.120659. Epub 2013 Jul 25. J Nucl Med. 2013. PMID: 23886728 Free PMC article.
-
[11C]-p-Hydroxyphenethylguanidine.2007 Jan 18 [updated 2008 Feb 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Jan 18 [updated 2008 Feb 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641521 Free Books & Documents. Review.
-
Dependence of cardiac 11C-meta-hydroxyephedrine retention on norepinephrine transporter density.J Nucl Med. 2006 Sep;47(9):1490-6. J Nucl Med. 2006. PMID: 16954558 Free PMC article.
-
Recent advances in radiotracers targeting norepinephrine transporter: structural development and radiolabeling improvements.J Neural Transm (Vienna). 2020 Jun;127(6):851-873. doi: 10.1007/s00702-020-02180-4. Epub 2020 Apr 9. J Neural Transm (Vienna). 2020. PMID: 32274584 Free PMC article. Review.
Cited by
-
LMI1195 PET imaging in evaluation of regional cardiac sympathetic denervation and its potential role in antiarrhythmic drug treatment.Eur J Nucl Med Mol Imaging. 2012 Dec;39(12):1910-9. doi: 10.1007/s00259-012-2204-y. Epub 2012 Aug 4. Eur J Nucl Med Mol Imaging. 2012. PMID: 22865199
-
(123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis.Eur J Nucl Med Mol Imaging. 2012 Oct;39(10):1609-17. doi: 10.1007/s00259-012-2187-8. Epub 2012 Jul 18. Eur J Nucl Med Mol Imaging. 2012. PMID: 22806059 Free PMC article.
-
[18F]Fluoro-Hydroxyphenethylguanidines: Efficient Synthesis and Comparison of Two Structural Isomers as Radiotracers of Cardiac Sympathetic Innervation.ACS Chem Neurosci. 2017 Jul 19;8(7):1530-1542. doi: 10.1021/acschemneuro.7b00051. Epub 2017 Mar 27. ACS Chem Neurosci. 2017. PMID: 28322043 Free PMC article.
-
Iodine-123 Metaiodobenzylguanidine (I-123 MIBG) in Clinical Applications: A Comprehensive Review.Pharmaceuticals (Basel). 2024 Nov 21;17(12):1563. doi: 10.3390/ph17121563. Pharmaceuticals (Basel). 2024. PMID: 39770405 Free PMC article. Review.
-
Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter.Pharmaceutics. 2023 Feb 17;15(2):690. doi: 10.3390/pharmaceutics15020690. Pharmaceutics. 2023. PMID: 36840011 Free PMC article.
References
-
- Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, et al. Radiolabeled adrenergic neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J. Nucl. Med. 1980;21:349–353. - PubMed
-
- Sisson JC, Frager MS, Valk TW, Gross MD, Swanson DP, et al. Scintigraphic localization of pheochromocytoma. N. Engl. J. Med. 1981;305:12–17. - PubMed
-
- Wieland DM, Mangner TJ, Inbasekaran MN, Brown LE, Wu J. Adrenal medulla imaging agents: a structure-distribution relationship study of radiolabeled aralkylguanidines. J. Med. Chem. 1984;27:149–155. - PubMed
-
- Tobes MC, Jaques S, Wieland DM, Sisson JC. Effect of uptake-one inhibitors on the uptake of norepinephrine and metaiodobenzylguanidine. J. Nucl. Med. 1985;26:897–907. - PubMed
-
- Bönisch H, Harder R. Binding of 3H-desipramine to the neuronal noradrenaline carrier of rat phaeochromocytoma cells (PC-12 cells) Naunyn-Schmiedeberg’s Arch. Pharmacol. 1986;334:403–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

