Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: a work in progress
- PMID: 17419950
- PMCID: PMC2912285
- DOI: 10.1016/S0065-230X(06)97012-2
Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: a work in progress
Abstract
Almost three decades ago, we presented a model where the extracellular matrix (ECM) was postulated to influence gene expression and tissue-specificity through the action of ECM receptors and the cytoskeleton. This hypothesis implied that ECM molecules could signal to the nucleus and that the unit of function in higher organisms was not the cell alone, but the cell plus its microenvironment. We now know that ECM invokes changes in tissue and organ architecture and that tissue, cell, nuclear, and chromatin structure are changed profoundly as a result of and during malignant progression. Whereas some evidence has been generated for a link between ECM-induced alterations in tissue architecture and changes in both nuclear and chromatin organization, the manner by which these changes actively induce or repress gene expression in normal and malignant cells is a topic in need of further attention. Here, we will discuss some key findings that may provide insights into mechanisms through which ECM could influence gene transcription and how tumor cells acquire the ability to overcome these levels of control.
Figures

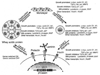
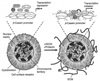
References
-
- Biggiogera M, Bottone MG, Scovassi AI, Soldani C, Vecchio L, Pellicciari C. Rearrangement of nuclear ribonucleoprotein (RNP)-containing structures during apoptosis and transcriptional arrest. Biol. Cell. 2004;96:603–615. - PubMed

