A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown
- PMID: 17419991
- PMCID: PMC2973840
- DOI: 10.1016/j.devcel.2007.01.019
A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown
Abstract
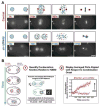
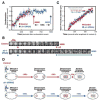
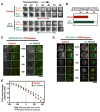
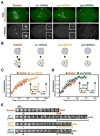
Aurora A kinase localizes to centrosomes and is required for centrosome maturation and spindle assembly. Here we describe a microtubule-independent role for Aurora A and centrosomes in nuclear envelope breakdown (NEBD) during the first mitotic division of the C. elegans embryo. Aurora A depletion does not alter the onset or kinetics of chromosome condensation, but dramatically lengthens the interval between the completion of condensation and NEBD. Inhibiting centrosome assembly by other means also lengthens this interval, albeit to a lesser extent than Aurora A depletion. By contrast, centrosomally nucleated microtubules and the nuclear envelope-associated motor dynein are not required for timely NEBD. These results indicate that mitotic centrosomes generate a diffusible factor, which we propose is activated Aurora A, that promotes NEBD. A positive feedback loop, in which an Aurora A-dependent increase in centrosome size promotes Aurora A activation, may temporally couple centrosome maturation to NEBD during mitotic entry.
Figures


Comment in
-
The centrosome opens the way to mitosis.Dev Cell. 2007 Apr;12(4):475-7. doi: 10.1016/j.devcel.2007.03.012. Dev Cell. 2007. PMID: 17419985
References
-
- Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. - PubMed
-
- Bar-Shira A, Pinthus JH, Rozovsky U, Goldstein M, Sellers WR, Yaron Y, Eshhar Z, Orr-Urtreger A. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002;62:6803–6807. - PubMed
-
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. - PubMed
-
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

