Nucleoplasmic calcium is required for cell proliferation
- PMID: 17420246
- PMCID: PMC2825877
- DOI: 10.1074/jbc.M700490200
Nucleoplasmic calcium is required for cell proliferation
Abstract
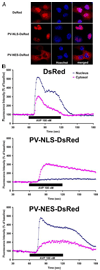
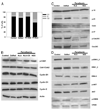
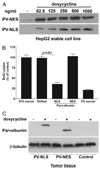
Ca(2+) signals regulate cell proliferation, but the spatial and temporal specificity of these signals is unknown. Here we use selective buffers of nucleoplasmic or cytoplasmic Ca(2+) to determine that cell proliferation depends upon Ca(2+) signals within the nucleus rather than in the cytoplasm. Nuclear Ca(2+) signals stimulate cell growth rather than inhibit apoptosis and specifically permit cells to advance through early prophase. Selective buffering of nuclear but not cytoplasmic Ca(2+) signals also impairs growth of tumors in vivo. These findings reveal a major physiological and potential pathophysiological role for nucleoplasmic Ca(2+) signals and suggest that this information can be used to design novel therapeutic strategies to regulate conditions of abnormal cell growth.
Figures




References
-
- Berridge MJ, Bootman MD, Roderick HL. Nat. Rev. Mol. Cell. Biol. 2003;4:517–529. - PubMed
-
- Clapham DE. Cell. 1995;80:259–268. - PubMed
-
- Kasai H, Augustine GJ. Nature. 1990;348:735–738. - PubMed
-
- Llinas R, Sugimori M, Silver RB. Science. 1992;256:677–679. - PubMed
-
- Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. J. Biol. Chem. 2005;280:33637–33644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

