Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis
- PMID: 17420270
- PMCID: PMC2118559
- DOI: 10.1084/jem.20062447
Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis
Erratum in
- J Exp Med. 2008 Mar 17;205(3):737
Abstract
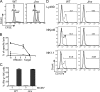
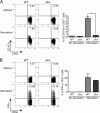
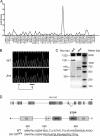
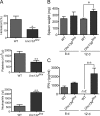
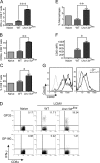
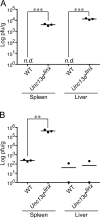
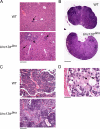
Mouse cytomegalovirus (MCMV) susceptibility often results from defects of natural killer (NK) cell function. Here we describe Jinx, an N-ethyl-N-nitrosourea-induced MCMV susceptibility mutation that permits unchecked proliferation of the virus, causing death. In Jinx homozygotes, activated NK cells and cytotoxic T lymphocytes (CTLs) fail to degranulate, although they retain the ability to produce cytokines, and cytokine levels are markedly elevated in the blood of infected mutant mice. Jinx was mapped to mouse chromosome 11 on a total of 246 meioses and confined to a 4.60-million basepair critical region encompassing 122 annotated genes. The phenotype was ascribed to the creation of a novel donor splice site in Unc13d, the mouse orthologue of human MUNC13-4, in which mutations cause type 3 familial hemophagocytic lymphohistiocytosis (FHL3), a fatal disease marked by massive hepatosplenomegaly, anemia, and thrombocytopenia. Jinx mice do not spontaneously develop clinical features of hemophagocytic lymphohistiocytosis (HLH), but do so when infected with lymphocytic choriomeningitis virus, exhibiting hyperactivation of CTLs and antigen-presenting cells, and inadequate restriction of viral proliferation. In contrast, neither Listeria monocytogenes nor MCMV induces the syndrome. In mice, the HLH phenotype is conditional, which suggests the existence of a specific infectious trigger of FHL3 in humans.
Figures
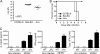
References
-
- Arase, H., E.S. Mocarski, A.E. Campbell, A.B. Hill, and L.L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296:1323–1326. - PubMed
-
- Lee, S.H., S. Girard, D. Macina, M. Busa, A. Zafer, A. Belouchi, P. Gros, and S.M. Vidal. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28:42–45. - PubMed
-
- Lee, S.H., A. Zafer, Y. de Repentigny, R. Kothary, M.L. Tremblay, P. Gros, P. Duplay, J.R. Webb, and S.M. Vidal. 2003. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J. Exp. Med. 197:515–526. - PMC - PubMed
-
- Brown, M.G., A.O. Dokun, J.W. Heusel, H.R. Smith, D.L. Beckman, E.A. Blattenberger, C.E. Dubbelde, L.R. Stone, A.A. Scalzo, and W.M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 292:934–937. - PubMed
-
- Crozat, K., P. Georgel, S. Rutschmann, N. Mann, X. Du, K. Hoebe, and B. Beutler. 2006. Analysis of the MCMV resistome by ENU mutagenesis. Mamm. Genome. 17:398–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

