Wilms' tumor protein Wt1 is an activator of the anti-Müllerian hormone receptor gene Amhr2
- PMID: 17420277
- PMCID: PMC1900060
- DOI: 10.1128/MCB.01780-06
Wilms' tumor protein Wt1 is an activator of the anti-Müllerian hormone receptor gene Amhr2
Abstract
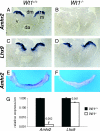
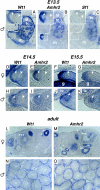
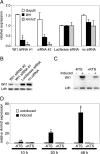
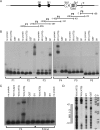
The Wilms' tumor protein Wt1 plays an essential role in mammalian urogenital development. WT1 mutations in humans lead to a variety of disorders, including Wilms' tumor, a pediatric kidney cancer, as well as Frasier and Denys-Drash syndromes. Phenotypic anomalies in Denys-Drash syndrome include pseudohermaphroditism and sex reversal in extreme cases. We have used cDNA microarray analyses on Wt1 knockout mice to identify Wt1-dependent genes involved in sexual development. The gene most dramatically affected by Wt1 inactivation was Amhr2, encoding the anti-Müllerian hormone (Amh) receptor 2. Amhr2 is an essential factor for the regression of the Müllerian duct in males, and mutations in AMHR2 lead to the persistent Müllerian duct syndrome, a rare form of male pseudohermaphroditism. Here we show that Wt1 and Amhr2 are coexpressed during urogenital development and that the Wt1 protein binds to the promoter region of the Amhr2 gene. Inactivation and overexpression of Wt1 in cell lines was followed by immediate changes of Amhr2 expression. The identification of Amhr2 as a Wt1 target provides new insights into the role of Wt1 in sexual differentiation and indicates, in addition to its function in early gonad development and sex determination, a novel function for Wt1, namely, in Müllerian duct regression.
Figures


Similar articles
-
The Wilms' tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Müllerian-inhibiting substance, and the androgen receptor.Clin Cancer Res. 1997 Dec;3(12 Pt 2):2571-80. Clin Cancer Res. 1997. PMID: 9815658
-
Effects of Denys-Drash syndrome point mutations on the DNA binding activity of the Wilms' tumor suppressor protein WT1.Biochemistry. 1996 Sep 17;35(37):12070-6. doi: 10.1021/bi960758o. Biochemistry. 1996. PMID: 8810912
-
Identification, expression, and regulation of anti-Müllerian hormone type-II receptor in the embryonic chicken gonad.Biol Reprod. 2014 May;90(5):106. doi: 10.1095/biolreprod.113.116491. Epub 2014 Mar 12. Biol Reprod. 2014. PMID: 24621923
-
The role of Wilms' tumor genes.J Med Invest. 1999 Aug;46(3-4):130-40. J Med Invest. 1999. PMID: 10687307 Review.
-
New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes.Am J Physiol Renal Physiol. 2008 Jul;295(1):F12-7. doi: 10.1152/ajprenal.00597.2007. Epub 2008 Apr 2. Am J Physiol Renal Physiol. 2008. PMID: 18385267 Review.
Cited by
-
Transcriptional regulation by the Wilms tumor protein, Wt1, suggests a role of the metalloproteinase Adamts16 in murine genitourinary development.J Biol Chem. 2013 Jun 28;288(26):18811-24. doi: 10.1074/jbc.M113.464644. Epub 2013 May 9. J Biol Chem. 2013. PMID: 23661704 Free PMC article.
-
Mechanisms of transcriptional regulation by WT1 (Wilms' tumour 1).Biochem J. 2014 Jul 1;461(1):15-32. doi: 10.1042/BJ20131587. Biochem J. 2014. PMID: 24927120 Free PMC article. Review.
-
Polycystic Ovary Syndrome Physiologic Pathways Implicated Through Clustering of Genetic Loci.J Clin Endocrinol Metab. 2024 Mar 15;109(4):968-977. doi: 10.1210/clinem/dgad664. J Clin Endocrinol Metab. 2024. PMID: 37967238 Free PMC article.
-
Amine oxidase copper-containing 1 (AOC1) is a downstream target gene of the Wilms tumor protein, WT1, during kidney development.J Biol Chem. 2014 Aug 29;289(35):24452-62. doi: 10.1074/jbc.M114.564336. Epub 2014 Jul 17. J Biol Chem. 2014. PMID: 25037221 Free PMC article.
-
Molecular genetics of Müllerian duct formation, regression and differentiation.Sex Dev. 2014;8(5):281-96. doi: 10.1159/000364935. Epub 2014 Jul 12. Sex Dev. 2014. PMID: 25033758 Free PMC article. Review.
References
-
- Allard, S., P. Adin, L. Gouedard, N. di Clemente, N. Josso, M. C. Orgebin-Crist, J. Y. Picard, and F. Xavier. 2000. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development 127:3349-3360. - PubMed
-
- Armstrong, J. F., K. Pritchard-Jones, W. A. Bickmore, N. D. Hastie, and J. B. Bard. 1993. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 40:85-97. - PubMed
-
- Baarends, W. M., J. T. Uilenbroek, P. Kramer, J. W. Hoogerbrugge, E. C. van Leeuwen, A. P. Themmen, and J. A. Grootegoed. 1995. Anti-Mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136:4951-4962. - PubMed
-
- Baarends, W. M., M. J. van Helmond, M. Post, P. J. van der Schoot, J. W. Hoogerbrugge, J. P. de Winter, J. T. Uilenbroek, B. Karels, L. G. Wilming, J. H. Meijers, et al. 1994. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Mullerian duct. Development 120:189-197. - PubMed
-
- Barakat, A. Y., Z. L. Papadopoulou, R. S. Chandra, C. E. Hollerman, and P. L. Calcagno. 1974. Pseudohermaphroditism, nephron disorder and Wilms' tumor: a unifying concept. Pediatrics 54:366-369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
