Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling
- PMID: 17420293
- PMCID: PMC2064116
- DOI: 10.1083/jcb.200609161
Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling
Abstract
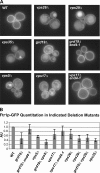
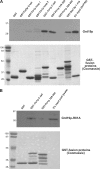
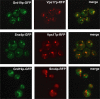
Amajor function of the endocytic system is the sorting of cargo to various organelles. Endocytic sorting of the yeast reductive iron transporter, which is composed of the Fet3 and Ftr1 proteins, is regulated by available iron. When iron is provided to iron-starved cells, Fet3p-Ftr1p is targeted to the lysosome-like vacuole and degraded. In contrast, when iron is not available, Fet3p-Ftr1p is maintained on the plasma membrane via an endocytic recycling pathway requiring the sorting nexin Grd19/Snx3p, the pentameric retromer complex, and the Ypt6p Golgi Rab GTPase module. A recycling signal in Ftr1p was identified and found to bind directly to Grd19/Snx3p. Retromer and Grd19/Snx3p partially colocalize to tubular endosomes, where they are physically associated. After export from the endosome, Fet3p-Ftr1p transits through the Golgi apparatus for resecretion. Thus, Grd19/Snx3p, functions as a cargo-specific adapter for the retromer complex, establishing a precedent for a mechanism by which sorting nexins expand the repertoire of retromer-dependent cargos.
Figures




References
-
- Askwith, C., D. Eide, A. Van Ho, P.S. Bernard, L. Li, S. Davis-Kaplan, D.M. Sipe, and J. Kaplan. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 76:403–410. - PubMed
-
- Bonifacino, J.S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7:568–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

