Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase
- PMID: 17420447
- PMCID: PMC1871862
- DOI: 10.1073/pnas.0701999104
Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase
Abstract
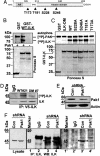
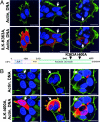
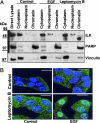
Integrin-linked kinase (ILK) is a phosphorylated protein that regulates physiological processes that overlap with those regulated by p21-activated kinase 1 (PAK1). Here we report the possible role of ILK phosphorylation by PAK1 in ILK-mediated signaling and intracellular translocation. We found that PAK1 phosphorylates ILK at threonine-173 and serine-246 in vitro and in vivo. Depletion of PAK1 decreased the levels of endogenous ILK phosphorylation in vivo. Mutation of PAK1 phosphorylation sites on ILK to alanine reduced cell motility and cell proliferation. Biochemical fractionation, confocal microscopy, and chromatin-interaction analyses of human cells revealed that ILK localizes predominantly in the cytoplasm but also resides in the nucleus. Transfection of MCF-7 cells with point mutants ILK-T173A, ILK-S246A, or ILK-T173A; S246A (ILK-DM) altered ILK localization. Selective depletion of PAK1 dramatically increased the nuclear and focal point accumulation of ILK, further demonstrating a role for PAK1 in ILK translocation. We also identified functional nuclear localization sequence and nuclear export sequence motifs in ILK, delineated an apparently integral role for ILK in maintaining normal nuclear integrity, and established that ILK interacts with the regulatory region of the CNKSR3 gene chromatin to negatively modulate its expression. Together, these results suggest that ILK is a PAK1 substrate, undergoes phosphorylation-dependent shuttling between the cell nucleus and cytoplasm, and interacts with gene-regulatory chromatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

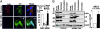

References
-
- Hannigan G, Troussard AA, Dedhar S. Nat Rev Cancer. 2005;5:51–63. - PubMed
-
- Esfandiarei M, Suarez A, Amaral A, Si X, Rahmani M, Dedhar S. Circ Res. 2006;99:354–361. - PubMed
-
- Wang B, Yurecko RS, Dedhar S, Cleary PP. Cell Microbiol. 2006;8:257–266. - PubMed
-
- Legate KR, Montanez E, Kudlacek O, Fassler R. Nat Rev Mol Cell Biol. 2006;7:20–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

