High-affinity single-domain binding proteins with a binary-code interface
- PMID: 17420456
- PMCID: PMC1871837
- DOI: 10.1073/pnas.0700149104
High-affinity single-domain binding proteins with a binary-code interface
Abstract
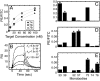
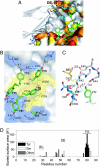
High degrees of sequence and conformation complexity found in natural protein interaction interfaces are generally considered essential for achieving tight and specific interactions. However, it has been demonstrated that specific antibodies can be built by using an interface with a binary code consisting of only Tyr and Ser. This surprising result might be attributed to yet undefined properties of the antibody scaffold that uniquely enhance its capacity for target binding. In this work we tested the generality of the binary-code interface by engineering binding proteins based on a single-domain scaffold. We show that Tyr/Ser binary-code interfaces consisting of only 15-20 positions within a fibronectin type III domain (FN3; 95 residues) are capable of producing specific binding proteins (termed "monobodies") with a low-nanomolar K(d). A 2.35-A x-ray crystal structure of a monobody in complex with its target, maltose-binding protein, and mutation analysis revealed dominant contributions of Tyr residues to binding as well as striking molecular mimicry of a maltose-binding protein substrate, beta-cyclodextrin, by the Tyr/Ser binary interface. This work suggests that an interaction interface with low chemical diversity but with significant conformational diversity is generally sufficient for tight and specific molecular recognition, providing fundamental insights into factors governing protein-protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lo Conte L, Chothia C, Janin J. J Mol Biol. 1999;285:2177–2198. - PubMed
-
- Fellouse FA, Li B, Compaan DM, Peden AA, Hymowitz SG, Sidhu SS. J Mol Biol. 2005;348:1153–1162. - PubMed
-
- Bogan AA, Thorn KS. J Mol Biol. 1998;280:1–9. - PubMed
-
- Fellouse FA, Barthelemy PA, Kelley RF, Sidhu SS. J Mol Biol. 2006;357:100–114. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

