The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey
- PMID: 17420491
- PMCID: PMC1839799
- DOI: 10.1503/cmaj.060410
The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey
Abstract
Background: Statistical tests for funnel-plot asymmetry are common in meta-analyses. Inappropriate application can generate misleading inferences about publication bias. We aimed to measure, in a survey of meta-analyses, how frequently the application of these tests would be not meaningful or inappropriate.
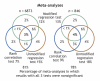
Methods: We evaluated all meta-analyses of binary outcomes with é 3 studies in the Cochrane Database of Systematic Reviews (2003, issue 2). A separate, restricted analysis was confined to the largest meta-analysis in each of the review articles. In each meta-analysis, we assessed whether criteria to apply asymmetry tests were met: no significant heterogeneity, I2 < 50%, é 10 studies (with statistically significant results in at least 1) and ratio of the maximal to minimal variance across studies > 4. We performed a correlation and 2 regression asymmetry tests and evaluated their concordance. Finally, we sampled 60 meta-analyses from print journals in 2005 that cited use of the standard regression test.
Results: A total of 366 of 6873 (5%) and 98 of 846 meta-analyses (12%) in the wider and restricted Cochrane data set, respectively, would have qualified for use of asymmetry tests. Asymmetry test results were significant in 7%-18% of the meta-analyses. Concordance between the 3 tests was modest (estimated k 0.33-0.66). Of the 60 journal meta-analyses, 7 (12%) would qualify for asymmetry tests; all 11 claims for identification of publication bias were made in the face of large and significant heterogeneity.
Interpretation: Statistical conditions for employing asymmetry tests for publication bias are absent from most meta-analyses; yet, in medical journals these tests are performed often and interpreted erroneously.
Figures

References
-
- Dickersin K, Min YI. Publication bias: the problem that won't go away. Ann N Y Acad Sci 1993;703:135-46. - PubMed
-
- Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet 1991;337:867-72. - PubMed
-
- Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 1998;279:281-6. - PubMed
-
- DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292:1363-4. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
