Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide
- PMID: 17420780
- PMCID: PMC2013978
- DOI: 10.1038/sj.bjp.0707230
Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide
Abstract
Background and purpose: There is major evidence for the strong bi-directional interrelation of parenchymal cell apoptosis and leukocyte accumulation and inflammation in acute liver injury. Therefore, the aim of this in vivo study was to investigate the anti-apoptotic and anti-inflammatory potential of antileukoproteinase (ALP) in a murine model of acute liver failure.
Experimental approach: C57BL/6J mice were given galactosamine (D-GalN) and E. coli lipopolysaccharide (LPS) followed by administration of saline or ALP. Besides survival rate, hepatic tissue damage and inflammatory response were analyzed by intravital fluorescence microscopy 6 hours after treatment. In addition, immunohistochemical analysis of NFkappaB-p65 and hepatocellular apoptosis, plasma levels of AST/ALT, TNF-alpha and IL-10 were determined.
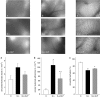
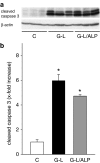
Key results: Administration of D-GalN/LPS provoked hepatic damage, including marked leukocyte recruitment and microvascular perfusion failure, as well as hepatocellular apoptosis and enzyme release. NFkappaB-p65 became increasingly detectable in hepatocellular nuclei, accompanied by a rise of TNF-alpha and IL-10 plasma levels. ALP markedly reduced intrahepatic leukocyte accumulation, nuclear translocation of NFkappaB and plasma levels of TNF-alpha and IL-10. Moreover, liver enzyme levels indicated the absence of necrotic parenchymal cell death. In contrast, ALP failed to block both apoptosis and caspase-3 levels and the mortality rate of ALP-treated animals was comparable to that of saline-treated mice.
Conclusions and implications: ALP could effectively prevent D-GalN/LPS-associated intrahepatic inflammatory responses by inhibition of NFkappaB activity, but not apoptosis-driven mortality. Thus, a protease-inactivating approach such as application of ALP seems to be inadequate in damaged liver where apoptosis represents the predominant mode of cell death.
Figures

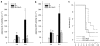

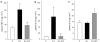
References
-
- Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187:S364–S369. - PubMed
-
- Abshagen K, Eipel C, Menger MD, Vollmar B. Comprehensive analysis of the regenerating mouse liver: an in vivo fluorescence microscopic and immunohistological study. J Surg Res. 2006;134:354–362. - PubMed
-
- Dijkstra G, Moshage H, Jansen PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease. Scand J Gastroenterol Suppl. 2002;236:37–41. - PubMed
-
- DiPiro JT. Cytokine networks with infection: mycobacterial infections, leishmaniasis, human immunodeficiency virus infection, and sepsis. Pharmacotherapy. 1997;17:205–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

