How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system
- PMID: 17420985
- PMCID: PMC3097047
- DOI: 10.1002/dvdy.21138
How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system
Abstract
Like the vertebrate enteric nervous system (ENS), the insect ENS consists of interconnected ganglia and nerve plexuses that control gut motility. However, the insect ENS lies superficially on the gut musculature, and its component cells can be individually imaged and manipulated within cultured embryos. Enteric neurons and glial precursors arise via epithelial-to-mesenchymal transitions that resemble the generation of neural crest cells and sensory placodes in vertebrates; most cells then migrate extensive distances before differentiating. A balance of proneural and neurogenic genes regulates the morphogenetic programs that produce distinct structures within the insect ENS. In vivo studies have also begun to decipher the mechanisms by which enteric neurons integrate multiple guidance cues to select their pathways. Despite important differences between the ENS of vertebrates and invertebrates, common features in their programs of neurogenesis, migration, and differentiation suggest that these relatively simple preparations may provide insights into similar developmental processes in more complex systems.
Copyright 2007 Wiley-Liss, Inc.
Figures
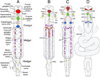
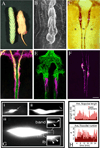
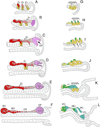

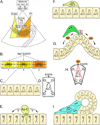

References
-
- Airaksinen MS, Holm L, Hatinen T. Evolution of the GDNF family ligands and receptors. Brain Behav Evol. 2006;68:181–190. - PubMed
-
- Anderson DJ. Stem cells and transcription factors in the development of the mammalian neural crest. Faseb J. 1994;8:707–713. - PubMed
-
- Anderson DJ. Lineages and transcription factors in the specification of vertebrate primary sensory neurons. Curr Opin Neurobiol. 1999;9:517–524. - PubMed
-
- Anderson RB, Turner KN, Nikonenko AG, Hemperly J, Schachner M, Young HM. The cell adhesion molecule L1 is required for chain migration of neural crest cells in the developing mouse gut. Gastroenterology. 2006;130:1221–1232. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

