TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis
- PMID: 17425404
- PMCID: PMC1851623
- DOI: 10.1371/journal.pmed.0040124
TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis
Abstract
Background: In multiple sclerosis, inflammation can successfully be prevented, while promoting repair is still a major challenge. Microglial cells, the resident phagocytes of the central nervous system (CNS), are hematopoietic-derived myeloid cells and express the triggering receptor expressed on myeloid cells 2 (TREM2), an innate immune receptor. Myeloid cells are an accessible source for ex vivo gene therapy. We investigated whether myeloid precursor cells genetically modified to express TREM2 affect the disease course of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis.
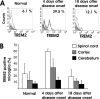
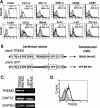
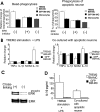
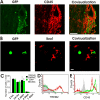
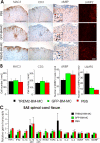
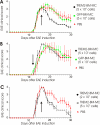
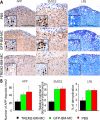
Methods and findings: EAE was induced in mice by immunization with a myelin autoantigen. Intravenous application of TREM2-transduced bone marrow-derived myeloid precursor cells at the EAE peak led to an amelioration of clinical symptoms, reduction in axonal damage, and prevention of further demyelination. TREM2-transduced myeloid cells applied intravenously migrated into the inflammatory spinal cord lesions of EAE-diseased mice, showed increased lysosomal and phagocytic activity, cleared degenerated myelin, and created an anti-inflammatory cytokine milieu within the CNS.
Conclusions: Intravenously applied bone marrow-derived and TREM2-tranduced myeloid precursor cells limit tissue destruction and facilitate repair within the murine CNS by clearance of cellular debris during EAE. TREM2 is a new attractive target for promotion of repair and resolution of inflammation in multiple sclerosis and other neuroinflammatory diseases.
Conflict of interest statement
Figures
Similar articles
-
Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis.J Neuroimmunol. 2007 Mar;184(1-2):92-9. doi: 10.1016/j.jneuroim.2006.11.032. Epub 2007 Jan 18. J Neuroimmunol. 2007. PMID: 17239445 Review.
-
Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2.J Exp Med. 2005 Feb 21;201(4):647-57. doi: 10.1084/jem.20041611. J Exp Med. 2005. PMID: 15728241 Free PMC article.
-
Expression of the HGF receptor c-met by macrophages in experimental autoimmune encephalomyelitis.Glia. 2010 Apr;58(5):559-71. doi: 10.1002/glia.20945. Glia. 2010. PMID: 19941340
-
Microglial cell activation and proliferation precedes the onset of CNS autoimmunity.J Neurosci Res. 2005 Aug 1;81(3):374-89. doi: 10.1002/jnr.20488. J Neurosci Res. 2005. PMID: 15959904
-
TREMs in the immune system and beyond.Nat Rev Immunol. 2003 Jun;3(6):445-53. doi: 10.1038/nri1106. Nat Rev Immunol. 2003. PMID: 12776204 Review.
Cited by
-
Differential roles of resident microglia and infiltrating monocytes in murine CNS autoimmunity.Semin Immunopathol. 2015 Nov;37(6):613-23. doi: 10.1007/s00281-015-0519-z. Epub 2015 Aug 4. Semin Immunopathol. 2015. PMID: 26240063 Review.
-
Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis.Stem Cells. 2012 Dec;30(12):2820-9. doi: 10.1002/stem.1218. Stem Cells. 2012. PMID: 22949039 Free PMC article.
-
Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke.J Cereb Blood Flow Metab. 2019 Oct;39(10):1906-1918. doi: 10.1177/0271678X18817282. Epub 2018 Dec 7. J Cereb Blood Flow Metab. 2019. PMID: 30523715 Free PMC article.
-
Microglial immunoreceptor tyrosine-based activation and inhibition motif signaling in neuroinflammation.Int J Alzheimers Dis. 2010 Jun 22;2010:587463. doi: 10.4061/2010/587463. Int J Alzheimers Dis. 2010. PMID: 20721346 Free PMC article.
-
The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells.J Biol Chem. 2010 Jan 29;285(5):2976-85. doi: 10.1074/jbc.M109.038398. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948717 Free PMC article.
References
-
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. - PubMed
-
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, et al. Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. - PubMed
-
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. - PubMed
-
- Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. - PubMed
-
- Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simburger E, et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J. 2005;19:647–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

