A novel bifunctional N-acetylglutamate synthase-kinase from Xanthomonas campestris that is closely related to mammalian N-acetylglutamate synthase
- PMID: 17425781
- PMCID: PMC1865377
- DOI: 10.1186/1471-2091-8-4
A novel bifunctional N-acetylglutamate synthase-kinase from Xanthomonas campestris that is closely related to mammalian N-acetylglutamate synthase
Abstract
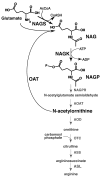
Background: In microorganisms and plants, the first two reactions of arginine biosynthesis are catalyzed by N-acetylglutamate synthase (NAGS) and N-acetylglutamate kinase (NAGK). In mammals, NAGS produces an essential activator of carbamylphosphate synthetase I, the first enzyme of the urea cycle, and no functional NAGK homolog has been found. Unlike the other urea cycle enzymes, whose bacterial counterparts could be readily identified by their sequence conservation with arginine biosynthetic enzymes, mammalian NAGS gene was very divergent, making it the last urea cycle gene to be discovered. Limited sequence similarity between E. coli NAGS and fungal NAGK suggests that bacterial and eukaryotic NAGS, and fungal NAGK arose from the fusion of genes encoding an ancestral NAGK (argB) and an acetyltransferase. However, mammalian NAGS no longer retains any NAGK catalytic activity.
Results: We identified a novel bifunctional N-acetylglutamate synthase and kinase (NAGS-K) in the Xanthomonadales order of gamma-proteobacteria that appears to resemble this postulated primordial fusion protein. Phylogenetic analysis indicated that xanthomonad NAGS-K is more closely related to mammalian NAGS than to other bacterial NAGS. We cloned the NAGS-K gene from Xanthomonas campestis, and characterized the recombinant NAGS-K protein. Mammalian NAGS and its bacterial homolog have similar affinities for substrates acetyl coenzyme A and glutamate as well as for their allosteric regulator arginine.
Conclusion: The close phylogenetic relationship and similar biochemical properties of xanthomonad NAGS-K and mammalian NAGS suggest that we have identified a close relative to the bacterial antecedent of mammalian NAGS and that the enzyme from X. campestris could become a good model for mammalian NAGS in structural, biochemical and biophysical studies.
Figures




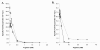

References
-
- Van de Casteele M, Demerez M, Legrain C, Glansdorff N, Pierard A. Pathways of arginine biosynthesis in extreme thermophilic archaeo- and eubacteria. J Gen Microbiol. 1990;136:1177–1183.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

