A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells
- PMID: 17426117
- PMCID: PMC1885678
- DOI: 10.1093/nar/gkm198
A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells
Abstract
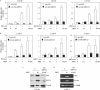
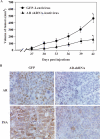
Although the vital role of the androgen receptor (AR) has been well demonstrated in primary prostate cancers, its role in the androgen-insensitive prostate cancers still remains unclear. Here, we used a small hairpin RNA approach to directly assess AR activity in prostate cancer cells. Reduction of AR expression in the two androgen-sensitive prostate cancer cell lines, LNCaP and LAPC4, significantly decreased AR-mediated transcription and cell growth. Intriguingly, in two androgen-insensitive prostate cell lines, LNCaP-C42B4 and CWR22Rv1, knockdown of AR expression showed a more pronounced effect on AR-induced transcription and cell growth than androgen depletion. Using cDNA microarrays, we also compared the transcriptional profiles induced by either androgen depletion or AR knockdown. Although a significant number of transcripts appear to be regulated by both androgen depletion and AR knockdown, we observed a subset of transcripts affected only by androgen depletion but not by AR knockdown, and vice versa. Finally, we demonstrated a direct role for AR in promoting tumor formation and growth in a xenograft model. Taken together, our results elucidate an important role for the AR in androgen-insensitive prostate cancer cells, and suggest that AR can be used as a therapeutic target for androgen-insensitive prostate cancers.
Figures




References
-
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–562. - PubMed
-
- Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. Androgen receptors in prostate cancer. J. Urol. 2003;170:1363–1369. - PubMed
-
- Gelmann EP. Molecular biology of the androgen receptor. J. Clin. Oncol. 2002;20:3001–3015. - PubMed
-
- Jenster G. The role of the androgen receptor in the development and progression of prostate cancer. Semin. Oncol. 1999;26:407–421. - PubMed
-
- Culig Z, Hobisch A, Bartsch G, Klocker H. Androgen receptor – an update of mechanisms of action in prostate cancer. Urol. Res. 2000;28:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

