An IAP retrotransposon in the mouse ADAMTS13 gene creates ADAMTS13 variant proteins that are less effective in cleaving von Willebrand factor multimers
- PMID: 17426255
- PMCID: PMC1924774
- DOI: 10.1182/blood-2007-01-070953
An IAP retrotransposon in the mouse ADAMTS13 gene creates ADAMTS13 variant proteins that are less effective in cleaving von Willebrand factor multimers
Abstract
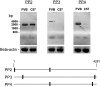
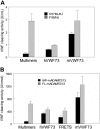
Severe deficiency of ADAMTS13, a von Willebrand factor (VWF)-cleaving metalloprotease, causes thrombotic thrombocytopenic purpura. When analyzed with VWF multimers, but not with an abbreviated VWF peptide (VWF73) as the substrate, the plasma ADAMTS13 activity levels of mouse strains segregated into a high and a low group that differed by approximately 10 fold. Low ADAMTS13 activity was detected in mice containing 2 alleles of intracisternal A-type particle (IAP) retrotransposon sequence in the ADAMTS13 gene. Molecular cloning of mouse ADAMTS13 identified 2 truncated variants (IAP-a and IAP-b) in the low-activity mice. Both of the IAP variants lacked the 2 carboxyl terminus thrombospondin type 1 repeat (TSR) and CUB domains of full-length ADAMTS13. The IAP-b variant also had splicing abnormalities affecting the spacer domain sequence and had miniscule enzymatic activity. Compared with full-length ADAMTS13, the IAP-a variant was approximately one ninth as active in cleaving VWF multimers but was only slightly less active in cleaving VWF73 peptide. Recombinant human ADAMTS13 was also less effective in cleaving VWF multimers than VWF73 when the C-terminal TSR sequence was deleted. In summary, the carboxyl terminus TSR sequence is important for cleaving VWF multimers. Assay results should be interpreted with caution when peptide substrates are used for analysis of variant ADAMTS13 proteins.
Figures




Similar articles
-
Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13).Blood. 2002 Nov 15;100(10):3626-32. doi: 10.1182/blood-2002-05-1397. Epub 2002 Jul 12. Blood. 2002. PMID: 12393399
-
Identification of strain-specific variants of mouse Adamts13 gene encoding von Willebrand factor-cleaving protease.J Biol Chem. 2004 Jul 16;279(29):30896-903. doi: 10.1074/jbc.M314184200. Epub 2004 May 10. J Biol Chem. 2004. PMID: 15136581
-
Interplay between ADAMTS13 and von Willebrand factor in inherited and acquired thrombotic microangiopathies.Semin Hematol. 2005 Jan;42(1):56-62. doi: 10.1053/j.seminhematol.2004.09.008. Semin Hematol. 2005. PMID: 15662617 Review.
-
Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura.J Mol Med (Berl). 2002 Oct;80(10):639-47. doi: 10.1007/s00109-002-0369-8. Epub 2002 Sep 5. J Mol Med (Berl). 2002. PMID: 12395148 Review.
-
[From gene to disease; congenital thrombotic thrombocytopenic purpura due to mutations in the ADAMTS13 gene].Ned Tijdschr Geneeskd. 2003 Dec 6;147(49):2422-4. Ned Tijdschr Geneeskd. 2003. PMID: 14694551 Review. Dutch.
Cited by
-
Mouse germ line mutations due to retrotransposon insertions.Mob DNA. 2019 Apr 13;10:15. doi: 10.1186/s13100-019-0157-4. eCollection 2019. Mob DNA. 2019. PMID: 31011371 Free PMC article. Review.
-
Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity.Blood. 2008 Sep 1;112(5):1713-9. doi: 10.1182/blood-2008-04-148759. Epub 2008 May 20. Blood. 2008. PMID: 18492952 Free PMC article.
-
A novel role for von Willebrand factor in the pathogenesis of experimental cerebral malaria.Blood. 2016 Mar 3;127(9):1192-201. doi: 10.1182/blood-2015-07-654921. Epub 2015 Oct 28. Blood. 2016. PMID: 26511133 Free PMC article.
-
Mouse endogenous retroviruses can trigger premature transcriptional termination at a distance.Genome Res. 2012 May;22(5):870-84. doi: 10.1101/gr.130740.111. Epub 2012 Feb 23. Genome Res. 2012. PMID: 22367191 Free PMC article.
-
N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity.Blood. 2009 Jan 22;113(4):929-35. doi: 10.1182/blood-2008-07-167775. Epub 2008 Nov 3. Blood. 2009. PMID: 18981290 Free PMC article.
References
-
- Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–985. - PubMed
-
- Uemura M, Tatsumi K, Matsumoto M, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–924. - PubMed
-
- Suzuki M, Murata M, Matsubara Y, et al. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem Biophys Res Commun. 2004;313:212–216. - PubMed
-
- Liu L, Choi H, Bernardo A, et al. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J Thromb Haemost. 2005;3:2536–2544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

