Regulation of COX-2 mediated signaling by alpha3 type IV noncollagenous domain in tumor angiogenesis
- PMID: 17426256
- PMCID: PMC1939900
- DOI: 10.1182/blood-2007-01-066282
Regulation of COX-2 mediated signaling by alpha3 type IV noncollagenous domain in tumor angiogenesis
Abstract
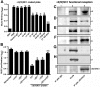
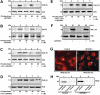
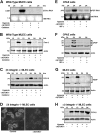
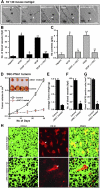
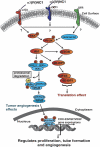
Human alpha3 chain, a noncollagenous domain of type IV collagen [alpha3(IV)NC1], inhibits angiogenesis and tumor growth. These biologic functions are partly attributed to the binding of alpha3(IV)NC1 to alphaVbeta3 and alpha3beta1 integrins. alpha3(IV)NC1 binds alphaVbeta3 integrin, leading to translation inhibition by inhibiting focal adhesion kinase/phosphatidylinositol 3-kinase/Akt/mTOR/4E-BP1 pathways. In the present study, we evaluated the role of alpha3beta1 and alphaVbeta3 integrins in tube formation and regulation of cyclooxygenase-2 (COX-2) on alpha3(IV)NC1 stimulation. We found that although both integrins were required for the inhibition of tube formation by alpha3(IV)NC1 in endothelial cells, only alpha3beta1 integrin was sufficient to regulate COX-2 in hypoxic endothelial cells. We show that binding of alpha3(IV)NC1 to alpha3beta1 integrin leads to inhibition of COX-2-mediated pro-angiogenic factors, vascular endothelial growth factor, and basic fibroblast growth factor by regulating IkappaBalpha/NFkappaB axis, and is independent of alphaVbeta3 integrin. Furthermore, beta3 integrin-null endothelial cells, when treated with alpha3(IV)NC1, inhibited hypoxia-mediated COX-2 expression, whereas COX-2 inhibition was not observed in alpha3 integrin-null endothelial cells, indicating that regulation of COX-2 by alpha3(IV)NC1 is mediated by integrin alpha3beta1. Our in vitro and in vivo findings demonstrate that alpha3beta1 integrin is critical for alpha3(IV)NC1-mediated inhibition of COX-2-dependent angiogenic signaling and inhibition of tumor progression.
Figures

References
-
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. - PubMed
-
- Distler O, Neidhart M, Gay RE, Gay S. The molecular control of angiogenesis. Int Rev Immunol. 2002;21:33–49. - PubMed
-
- Tryggvason K, Hoyhtya M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24:209–218. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

