The micromachinery of mechanotransduction in hair cells
- PMID: 17428178
- PMCID: PMC2865174
- DOI: 10.1146/annurev.neuro.29.051605.112917
The micromachinery of mechanotransduction in hair cells
Abstract
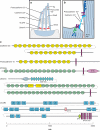
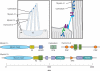
Mechanical stimuli generated by head movements and changes in sound pressure are detected by hair cells with amazing speed and sensitivity. The mechanosensitive organelle, the hair bundle, is a highly elaborated structure of actin-based stereocilia arranged in precise rows of increasing height. Extracellular linkages contribute to its cohesion and convey forces to mechanically gated channels. Channel opening is nearly instantaneous and is followed by a process of sensory adaptation that keeps the channels poised in their most sensitive range. This process is served by motors, scaffolds, and homeostatic mechanisms. The molecular constituents of this process are rapidly being elucidated, especially by the discovery of deafness genes and antibody targets.
Figures

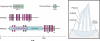

References
-
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 2005a;14:3921–32. - PubMed
-
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum. Mol. Genet. 2005b;14:347–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

