Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors
- PMID: 17428292
- PMCID: PMC2288551
- DOI: 10.1111/j.1530-0277.2007.00394.x
Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors
Abstract
Background: Rodent models of chronic alcohol exposure are typically constrained to relatively short periods of forced ethanol due to the lifespan of these animals. Nonhuman primate models, particularly those employing long-term self-administration, are conceptually more similar to human alcoholic individuals.
Methods: We performed whole-cell patch clamp recordings on acutely dissociated amygdala neurons isolated from cynomolgus macaque coronal temporal lobe slices. Slices were prepared from control monkeys or monkeys allowed to self-administer oral ethanol for 18 months. Flunitrazepam and acute ethanol modulation of currents gated by exogenous gamma-aminobutyric acid (GABA) application was assessed in these isolated neurons. Complementary experiments were performed on amygdala total RNA using quantitative real-time reverse transcription/polymerase chain reaction to understand potential ethanol-dependent adaptations to subunit composition.
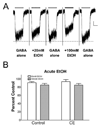
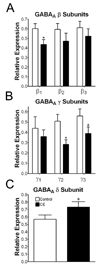
Results: Gamma-aminobutyric acid-gated currents from ethanol-exposed macaque amygdala neurons exhibited reduced modulation by flunitrazepam compared with control neurons. However, this was specific for benzodiazepines as the modest inhibition of GABA-gated currents by acute ethanol was not affected by the chronic ethanol consumption. We also measured mRNA expression levels for the beta, gamma, and delta subunits in total amygdala RNA isolated from control and ethanol-drinking animals. beta1 and gamma2 expression was significantly reduced in samples from ethanol-exposed amygdala.
Conclusions: Our findings demonstrate that chronic ethanol self-administration reduces the benzodiazepine sensitivity of amygdala GABA(A) receptors. This reduced sensitivity may be the result of decreased expression of an amygdala gamma subunit. These findings suggest that, while rodent and nonhuman primate models of chronic ethanol exposure share many characteristics, the specific molecular adaptations associated with the amygdala GABAergic system may not be identical.
Figures

References
-
- Bao K, Middaugh LD, Becker HC, Shepherd CL. Effects of Ro 15–4513 and ethanol on operant behavior of male and female C57BL/6 mice. Alcohol. 1992;9(3):193–198. - PubMed
-
- Becker HC, Anton RF, De Trana C, Randall CL. Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci. 1985;37(14):1293–1300. - PubMed
-
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in ice with a history of multiple withdrawal experience. Pharmacol Biochem Behav. 1997;57(1–2):179–183. - PubMed
-
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience electively alters sensitivity to different chemoconvulsant drugs in mice. psychopharmacology (Berl) 1998;139(1–2):145–153. - PubMed
-
- Benke D, Honer M, Michel C, Mohler H. GABAA receptor subtypes differentiated by their gamma-subunit variants: prevalence, pharmacology and subunit architecture. Neuropharmacology. 1996;35(9–10):1413–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

